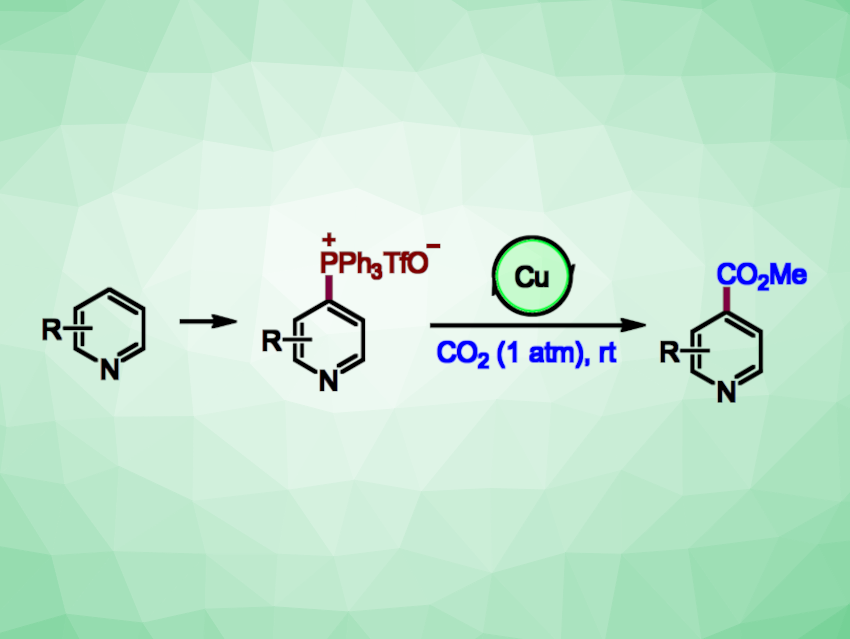
Pyridines are often found, e.g., in bioactive compounds, ligands, or functional materials. New methods for the site-selective functionalization of pyridines are interesting research targets. Pyridine-4-carboxylic acid (isonicotinic acid) derivatives, for example, are part of the structure of many bioactive molecules. The carboxylation of pyridines using CO2 could be an attractive approach to the synthesis of these compounds, but can be challenging to achieve selectively and under mild conditions.
Baiquan Wang, Nankai University, Tianjin, China, and Shanghai Institute of Organic Chemistry, Chinese Academy of Sciences, and colleagues have developed a practical method for the C-4 selective carboxylation of pyridines via a one-pot protocol. The process involves a known C−H phosphination, followed by a novel copper-catalyzed carboxylation of the resulting phosphonium salts with CO2 (general reactions pictured). The phosphonium salts were reacted with CO2 (1 atm) in the presence of CuCl as a catalyst, N,N,N‘,N‘-tetramethylethylenediamine (TMEDA) as a ligand, ZnEt2 as a reductant, and dimethylacetamide (DMA) as a solvent at room temperature.
The desired isonicotinic acid derivatives were obtained in moderate to high yields. The team successfully used the method for the late-stage carboxylation of complex, drug-like compounds. The reaction has a good functional group tolerance and can be performed an a gram scale. According to the researchers, it may be useful in drug design and discovery.
- Copper‐Catalyzed C4‐selective Carboxylation of Pyridines with CO2 via Pyridylphosphonium Salts,
Shibiao Tang, Zezhao Liu, Jiakai Zhang, Bin Li, Baiquan Wang,
Angew. Chem. Int. Ed. 2024.
https://doi.org/10.1002/anie.202318572