Plastic waste is an important environmental problem. Biodegradable polymers could help to address this issue. Polyhydroxyalkanoates (PHAs), for example, show inherent biodegradability. Thioester analogs of PHAs, i.e., polythioesters (PTEs), could also have useful properties. However, the properties of this type of polymer can heavily depend on their stereochemistry, and the synthesis of isotactic polythioesters can be challenging.
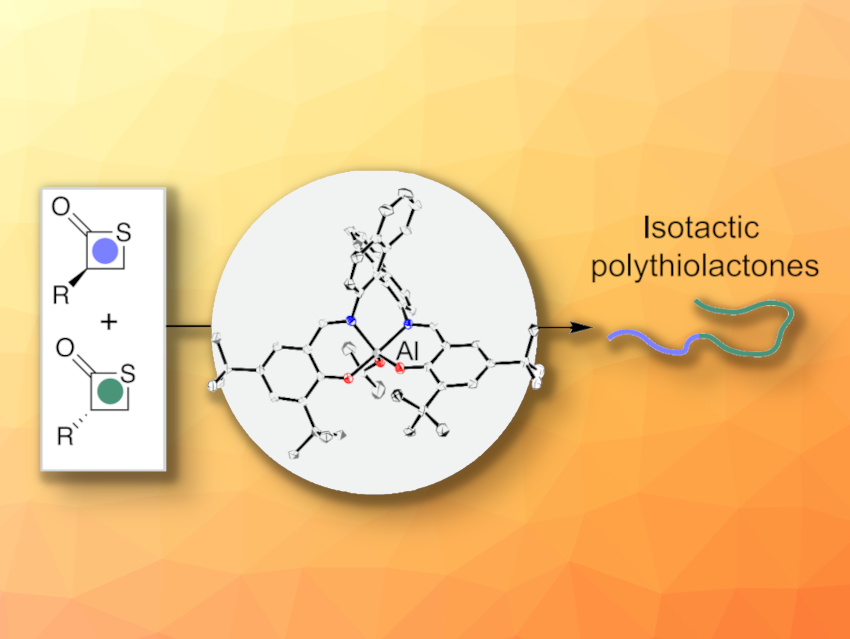
Jian-Bo Zhu, Sichuan University, Chengdu, China, and colleagues have developed an approach for the synthesis of perfectly isotactic PTEs via a stereocontrolled ring-opening polymerization. The team used a chiral binaphthalene-salen aluminium (SalBinam-Al) catalyst (pictured above in the center) to promote the polymerization of rac-α-substituted-β-propiothiolactones (general structure pictured above on the left) with kinetic resolution. They polymerized rac-α-benzyl-β-propiothiolactone (rac-BTL) and rac-α-isopropyl-β-propiothiolactone (rac-PTL).
Under these conditions, the researchers obtained perfectly isotactic polythioesters with molecular weights of up to 276 kDa. The developed approach also enabled the researchers to isolate enantiopure monomers via hydrolysis of the polymer. The product could then be further converted to (S)-2-benzyl-3-mercapto-propanoic acid, a useful building block in pharmaceutical chemistry. Overall, the work provides access to isotactic polythioesters that could be useful for the discovery of sustainable plastics for different applications.
- Kinetic Resolution Polymerization Enabled Chemical Synthesis of Perfectly Isotactic Polythioesters,
Kun Li, Jing-Liang Cheng, Meng-Yuan Wang, Wei Xiong, Hao-Yi Huang, Liang-Wen Feng, Zhongzheng Cai, Jian-Bo Zhu,
Angew. Chem. Int. Ed. 2024.
https://doi.org/10.1002/anie.202405382