Aromaticity-driven reactions can be useful in organic synthesis. π-Aromaticity is an important driving force in directing the synthesis of organic compounds. In contrast, reactions driven by σ-aromaticity are rare. Metallacyclopropenes, for example, can have σ-aromatic character. The synthesis and transformation of small heterocycles such as metallacyclopropenes can be valuable in both synthetic and catalytic chemistry. Due to ring strain effects, small metallacycles are prone to ring expansion or ring opening. However, ring contraction reactions are challenging to achieve for already small four-membered rings.
Yu-Mei Lin, Xiamen University, China, Haiping Xia, Xiamen University and Southern University of Science and Technology, Shenzhen, China, and colleagues have developed a strategy driven by π- and σ-aromaticity to achieve an unprecedented ring contraction of a metallacyclobutadiene to a metallacyclopropene (general structures pictured). The team first prepared an osmapentalyne by reacting OsCl2(PPh3)3 with a multiyne. The four-membered ring, an osmacyclobutadiene, was then introduced by a reaction with a terminal alkyne.
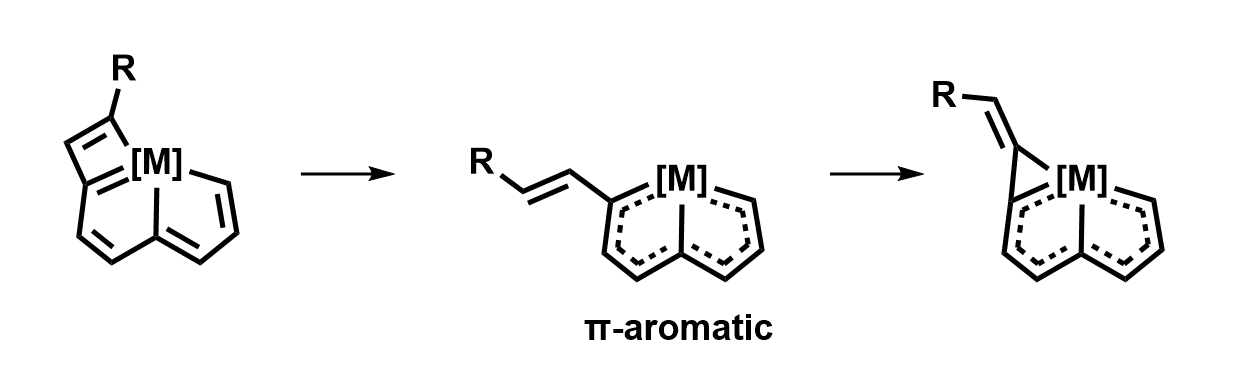
The resulting product (pictured above on the left) has an antiaromatic cyclobutadiene-type unit and an otherwise non-aromatic skeleton. When it was treated with CF3COOH, the team observed a ring contraction of the metallacyclobutadiene to a metallacyclopropene. The ring-opening-reclosing mechanism (pictured above) involves the opening of the π-antiaromatic metallacyclobutadiene to give a π-aromatic intermediate, followed by ring closure to generate a σ-aromatic metallacyclopropene. These findings demonstrate the importance of using aromaticity to realize challenging reactions.
- Ring contraction of metallacyclobutadiene to metallacyclopropene driven by π- and σ-aromaticity relay,
Kaiyue Zhuo, Yanan Liu, Kaidong Ruan, Yuhui Hua, Yu-Mei Lin, Haiping Xia,
Nat. Synth. 2022.
https://doi.org/10.1038/s44160-022-00194-2


![A Path to Substituted Bicyclo[2.1.1]hexanones](https://www.chemistryviews.org/wp-content/uploads/2024/10/1substitutedbicyclo211hexan2ones_2024-125x94.png)
