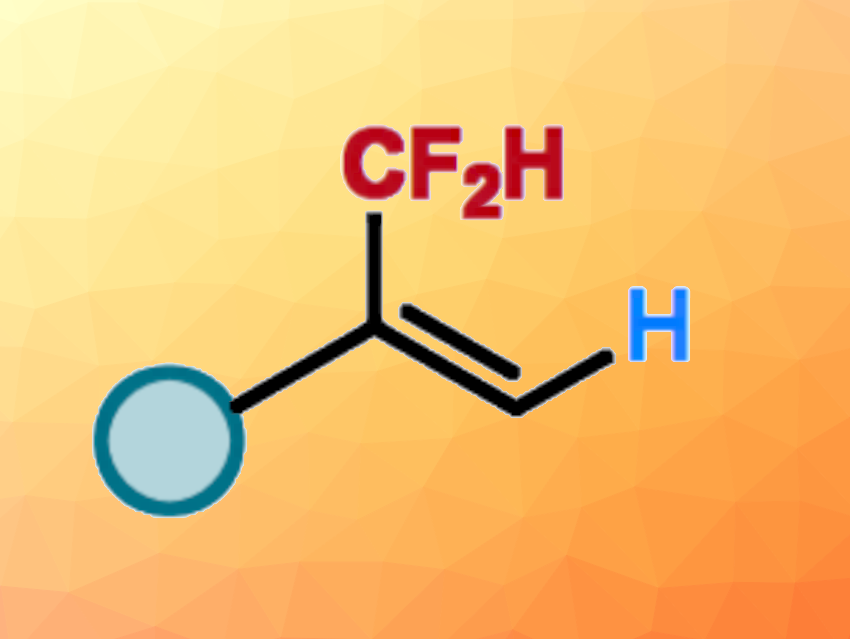
Introducing difluoromethyl (CF2H) groups can be useful to change the physicochemical and biological properties of a molecule, e.g., in pharmaceutical chemistry. One approach to this is the hydrodifluoromethylation of unsaturated bonds. In contrast to hydrodifluoromethylations of alkenes, the hydrodifluoromethylation of readily accessible alkynes for the direct assembly of CF2H-substituted alkenes is underexploited.
Lingling Chu, Donghua University, Shanghai, China, and colleagues have developed a Markovnikov-selective hydrodifluoromethylation of alkynes with BrCF2H using nickel catalysis (pictured below). The team used NiCl2•(PCy3)2 (PCy3 = tricyclohexyl phosphine) as a precatalyst, 4,4′-di-tert-butyl-2,2′-bipyridine (dtbbpy) as a ligand, (EtO)2MeSiH as a silane reactant, K2HPO4•3H2O as a base, and a mixture of ethyl acetate and N-methyl-2-pyrrolidone (NMP) as the solvent. The reactions were performed at 30 °C.
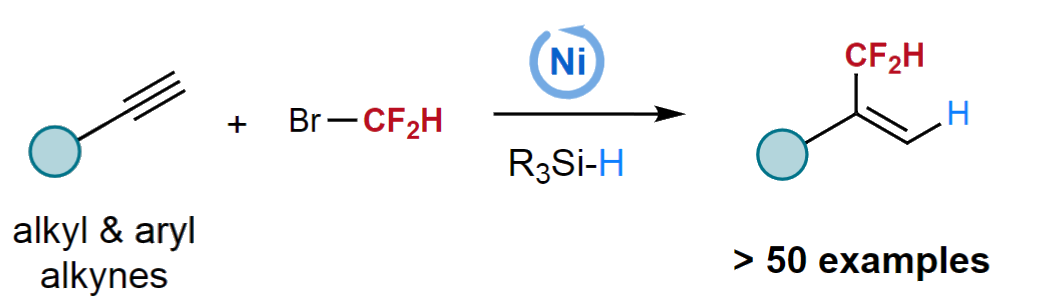
Under these conditions, a variety of aliphatic and (hetero)aryl alkynes were reacted with BrCF2H to give branched CF2H-substituted alkenes with exclusive regioselectivity and good yields. In addition to BrCF2H, α-chloro-α,α-difluoroacetates and α-chloro-α,α-difluoroacetamides could also be used as coupling partners to give α-difluoroalkyl-substituted alkenes in moderate yields. The researchers propose a reaction mechanism that involves a regioselective migratory insertion of the alkyne into a nickel hydride species, followed by an oxidative addition of BrCF2H and a reductive elimination to release the product.
- Nickel‐Catalyzed Markovnikov‐Selective Hydrodifluoromethylation of Alkynes Using BrCF2H,
Shiwei Pan, Fan Chen, Yanyan Zhang, Liang Shao, Lingling Chu,
Angew. Chem. Int. Ed. 2023.
https://doi.org/10.1002/anie.202305426