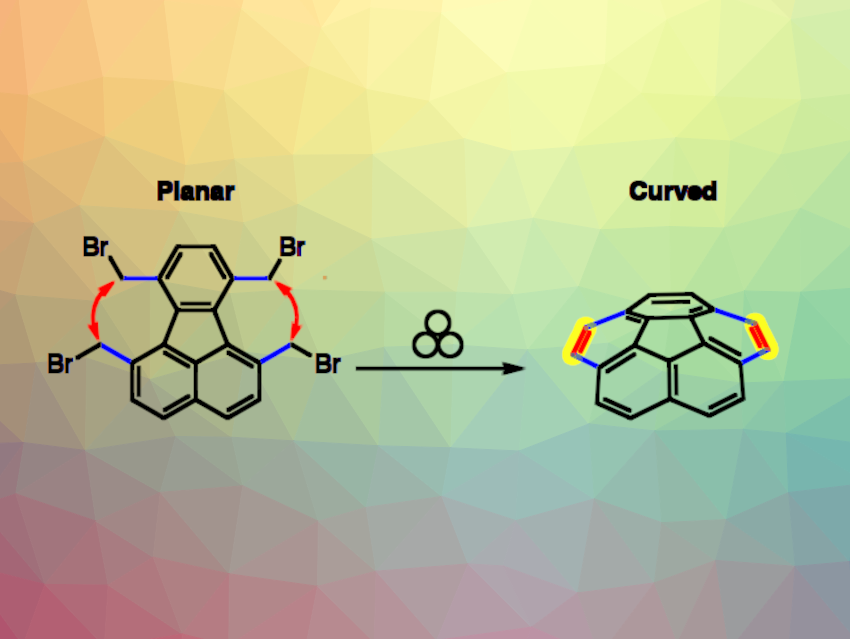
Corannulene is the smallest fragment of the fullerene C60 that retains a curved geometry. It is a useful building block in the synthesis of a variety of carbon-rich organic materials.
Mihaiela Corina Stuparu, Nanyang Technological University (NTU), Singapore, and colleagues have developed a mechanochemical method in which a suitable precursor can be ground in a ball mill to yield corannulene in high isolated yields (reaction pictured). This route, therefore, does not require the use of toxic or expensive organic solvents and enhances the sustainability of the synthesis.
The team first prepared the precursor starting from 3,8-dimethylacenaphthenequinone, which underwent a Knoevenagel condensation with 3-pentanone. The resulting intermediate was reacted with norbornadiene in a Diels–Alder reaction to give 1,6,7,10-tetramethylfluoranthene. Benzylic bromination then gave tetrabromomethylfluoranthene, the desired precursor. Milling this precursor in a stainless-steel jar with a steel ball in the presence of Ba(OH)2·8H2O, Na2SO4, and tetrabutylammonium chloride (TBACl) for 30 min then gave corannulene.
A crucial aspect of this work is the effort to scale up the mechanochemical synthesis. For this, the team used a planetary mill in which the milling jars spin counter-directionally to a spinning disc. Using this setup, they obtained 15 g of corannulene in a single milling cycle with an isolated yield of 90 %. These results could provide a strong motivation to explore the rational synthesis of other curved nanocarbons such as fullerenes and carbon nanotubes using mechanochemistry.
- Mechanochemical synthesis of corannulene: Scalable and efficient preparation of a curved polycyclic aromatic hydrocarbon under ball milling conditions,
Gábor Báti, Shoba Laxmi, Mihaiela Corina Stuparu,
ChemSusChem 2023.
https://doi.org/10.1002/cssc.202301087