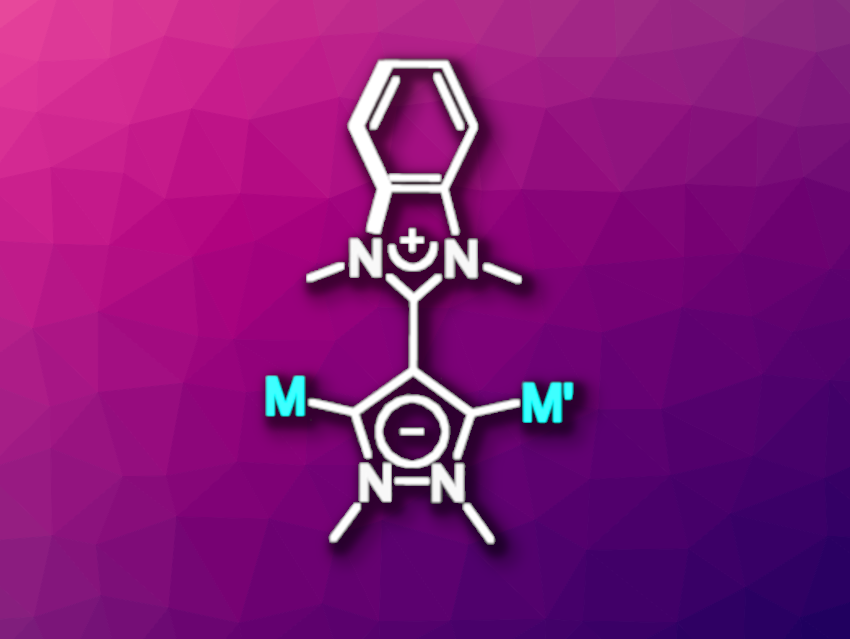
N-heterocyclic carbenes (NHCs) are an important class of ligands, often used in transition-metal catalysis, for example. Multitopic NHCs, i.e., NHCs with more than one carbene center, are interesting in this context. Janus-type dicarbenes, for example, have two carbene centers that face in opposite directions. Creating fully neutral dicarbenes of this type in which both carbene centers are situated in a single ring is interesting, because the resulting short intermetallic distances could allow for electronic communication between the metal centers. However, this can be challenging because the corresponding precursor generally needs to “fit” two positive charges into a single ring.
Han Vinh Huynh, National University of Singapore, Republic of Singapore, and colleagues have found a way around this restriction: The team used a dicationic precursor with one positive group outside of the ring, namely, a pyrazolium ring bound to a benzimidazolium unit. A double deprotonation then leads to a mesoionic Janus-type dicarbene, i.e., an overall neutral structure with both a positive and negative charge, which are delocalized. This Janus-type dicarbene can form homo- and heterobimetallic complexes (general structure pictured).
The team first prepared the dicationic precursor starting from 1-methyl-1H-pyrazole-4-carboxaldehyde and o-phenylenediamine via condensation and stepwise methylation. When the resulting precursor was reacted with silver oxide, a dinuclear palladium complex, and tetrabutylammonium bromide (TBAB), a dipalladium complex of the desired Janus-type dicarbene was formed. From the same precursor, the team also synthesized a heterobimetallic palladium(II)/gold(I) complex via an intermediate monopalladium complex. This product could then be oxidized to give a palladium(II)/gold(III) complex. The researchers performed preliminary catalytic studies and found that the Janus-type dicarbene dipalladium complex showed promising activity for the 2,5-diarylation of N-substituted pyrroles.
- Design of a Mesoionic Janus-type Dicarbene,
Jia Nuo Leung, Han Vinh Huynh,
J. Am. Chem. Soc. 2024.
https://doi.org/10.1021/jacs.3c13284