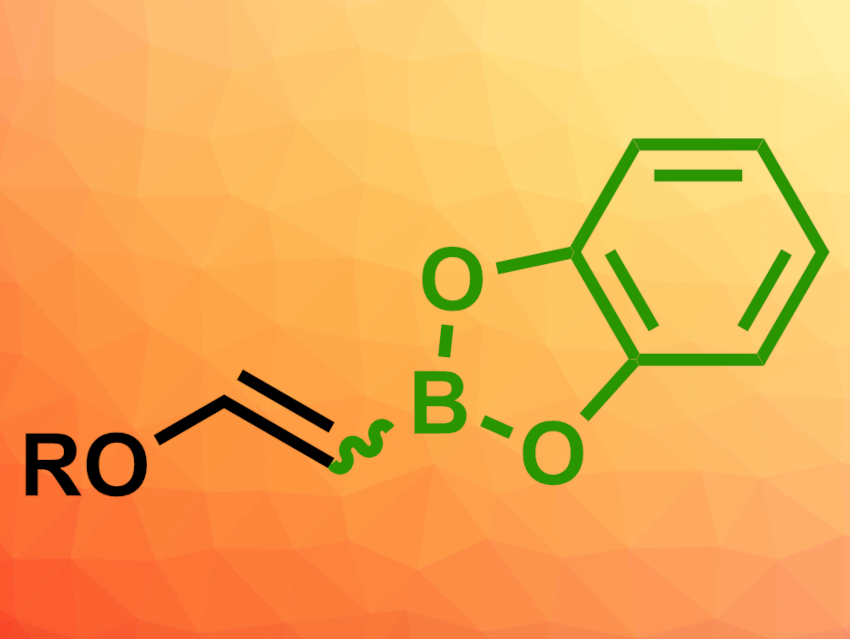
Organoboron compounds are useful intermediates in organic synthesis. Alkenyl boronates, in particular, are often used. They can be prepared via the hydroboration of alkynes, but this approach can suffer from poor functional group tolerance and problems with selectivity.
Frédéric-Georges Fontaine, Université Laval, Quebec City, Canada, and colleagues have developed an approach for the metal-free C–H borylation of electron-rich alkenes to give alkenyl boronates (example pictured). The team reacted different enol ethers, silyl enol ethers, or enamines with 2-furyl–Bcat (HBCat = catecholborane) in the presence of a 2-mercaptoimidazole as the catalyst in benzene at 110 °C.
The desired alkenyl boronates were obtained in high yields for enol ether substrates and generally low to moderate yields for silyl enol ether substrates. For enamines, the yield depends strongly on the substrate, ranging from 0 % to 82 % for the tested substrates. The catecholboronate products were found to be challenging to isolate, but can easily be used in further reactions in a one-pot approach. The strategy shows a good functional group tolerance, which could make it a versatile tool for the functionalization of electron-rich alkenes.
- A Metal-Free Approach for the C–H Activation and Transfer Borylation of Electron-Rich Alkenes,
Vincent Desrosiers, Samantha M. Knight, Frédéric-Georges Fontaine,
ACS Catal. 2022.
https://doi.org/10.1021/acscatal.2c04305