Carbon dioxide from the combustion of fossil fuels is the primary contributor to climate change. Capturing CO2 and using it, e.g., as a chemical feedstock could help to alleviate this problem. CO2 can be converted into cyclic carbonates, for example. Existing heterogeneous catalysts for this type of reaction often require high temperatures and/or high CO2 pressures. The development of improved catalysts is, thus, an interesting research target.
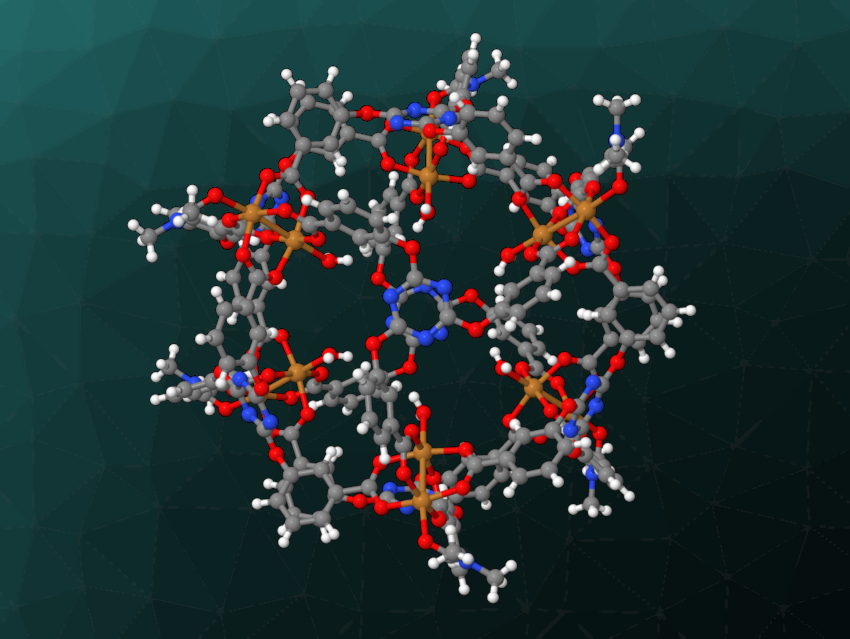
Lin Liu, Zheng-Bo Han, Liaoning University, Shenyang, China, and colleagues have synthesized a copper-based metal–organic polyhedron (MOP, pictured) with a high specific surface area and high CO2 uptake. This MOP could be used as a heterogeneous catalyst for the chemical fixation of CO2.
The team synthesized the metal–organic polyhedron by reacting Cu(NO3)2·3H2O with 2,4,6-tris(3-carboxyphenoxy)-1,3,5-triazine (H3TCPT) in a mixture of dimethylformamide (DMF) and CH3CN at 75 °C. They obtained solvent adducts of [Cu12(TCPT)8(H2O)6(DMF)6] as blue crystals. The copper-based polyhedra contain square-planar paddle-wheel-type Cu(II) clusters bridged by TCPT ligands.
The synthesized MOP has a high specific surface area of 407 m2g–1 and high CO2 uptake that corresponds to about 28 adsorbed CO2 molecules per MOP unit. It also has a high density of Lewis-acidic copper sites, which is promising for applications in catalysis. The team used it as a heterogeneous catalyst for the cycloaddition of CO2 with epoxides to give cyclic carbonates and observed good performance under ambient conditions.
- A [(M2)6L8] metal–organic polyhedron with high CO2 uptake and efficient chemical conversion of CO2 under ambient conditions,
Li-Hua Liu, Lin Liu, Hao-Ran Chi, Chen-Ning Li, Zheng-Bo Han,
Chem. Commun. 2022.
https://doi.org/10.1039/d2cc01734b