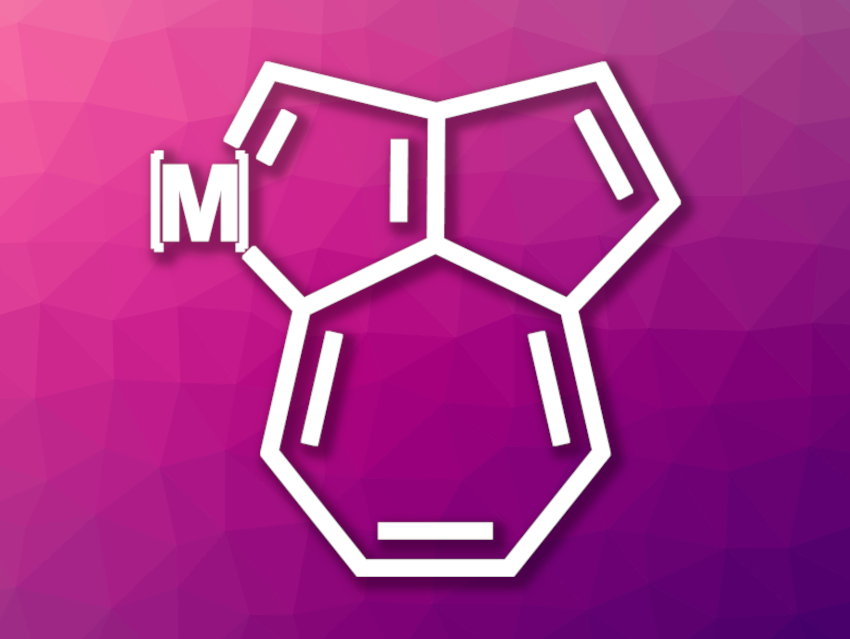
Azulene is an isomer of naphthalene with fused five- and seven-membered rings. Azulene-based conjugated systems are interesting research targets due to their unusual structures and photophysical properties. The incorporation of a transition metal into the azulene skeleton could provide an opportunity to combine dπ-pπ and pπ-pπ conjugated properties, but no such metallaazulene skeleton had been reported so far.
Yu-Mei Lin, Xiamen University, China, Haiping Xia, Xiamen University and Southern University of Science and Technology, China, and colleagues have developed a convenient [5+2] annulation strategy that can be used to construct an uncommon metal-containing [5-5-7] skeleton termed a metalla-dual-azulene (MDA, simplified structure pictured). This structure can be viewed as a cross between a metallaazulene and an organic azulene, both sharing the seven-membered ring. The team first prepared precursors for the [5 + 2] annulations from diynes and [Ir(CH3CN)(CO)(PPh3)2]BF4. The precursors were then treated with different alkynes in the presence of AgBF4 to obtain a range of the desired iridium-based metalla-dual-azulenes.
The replacement of a carbon atom by a transition metal in the [5-5-7] skeleton causes the two azulene fragments to show completely different reactivities. The organic azulene side allows for the installation of various substituents at the seven-membered ring through nucleophilic addition, which allows fine-tuning of the compound’s electronic properties. On the metal-functionalized side, a demetalation of the metallaazulene unit occurs when it reacts with nBu4NF, which enables the highly selective recognition of fluoride anions and results a noticeable color change. Overall, the synthesized polycyclic system could be suitable for applications, e.g., in photoelectric and sensing materials.
- Synthesis of metalla-dual-azulenes with fluoride ion recognition properties,
Hai-Cheng Liu, Kaidong Ruan, Kexin Ma, Jiawei Fei, Yu-Mei Lin, Haiping Xia,
Nat. Commun. 2023.
https://doi.org/10.1038/s41467-023-41250-5