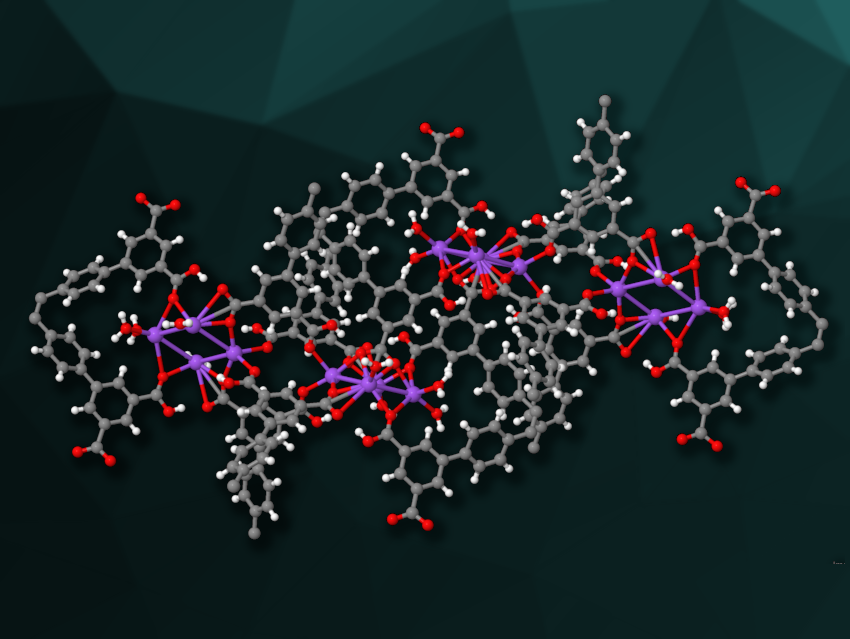
Metal–organic frameworks (MOFs) are crystalline, porous materials composed of metal centers and organic linkers. They have applications, e.g., in catalysis or gas separation. MOFs are most often based on transition metals or lanthanides. The synthesis of MOFs with permanent porosity based on alkali metals is challenging in comparison. Sodium-based MOFs, in particular, could be useful due to their low specific weight.
Hao Wang, Shenzhen Polytechnic University, Guangdong, China, and colleagues have synthesized a microporous, sodium-based MOF, called HIAM-111 (pictured). The team used the octacarboxylate linker 4′,4”’,4””’,4”””’-(ethene-1,1,2,2-tetrayl)tetrakis(([1,1′-biphenyl]-3,5-dicarboxylic acid)) (H8ETTBPDC) to prepare the MOF via a solvothermal reaction of NaCl with the linker in a mixture of water and N,N-dimethylformamide (DMF) at 120 °C.
The product was characterized using single-crystal X-ray diffraction, and the team found that the MOF features tetranuclear Na4(COO)8 building units. The linker’s eight carboxylate groups are connected to Na4 clusters in either monodentate or bidentate binding modes.
The MOF is robust and highly porous, with an estimated specific surface area of 1,561 m2/g—a much higher value than those found for previously known sodium-based MOFs. The researchers found that the material can be used for gas separation, namely, for purifying C2H4 from mixtures of C2H2, C2H4, and C2H6. Overall, the work could provide useful insights for the design of porous, alkali metal-based MOFs
- An Octacarboxylate-Linked Sodium Metal–Organic Framework with High Porosity,
Jiafeng Miao, Wells Graham, Jiaqi Liu, Ena Clementine Hill, Lu-Lu Ma, Saif Ullah, Hai-Lun Xia, Fu-An Guo, Timo Thonhauser, Davide M. Proserpio, Jing Li, Hao Wang,
J. Am. Chem. Soc. 2023.
https://doi.org/10.1021/jacs.3c11260