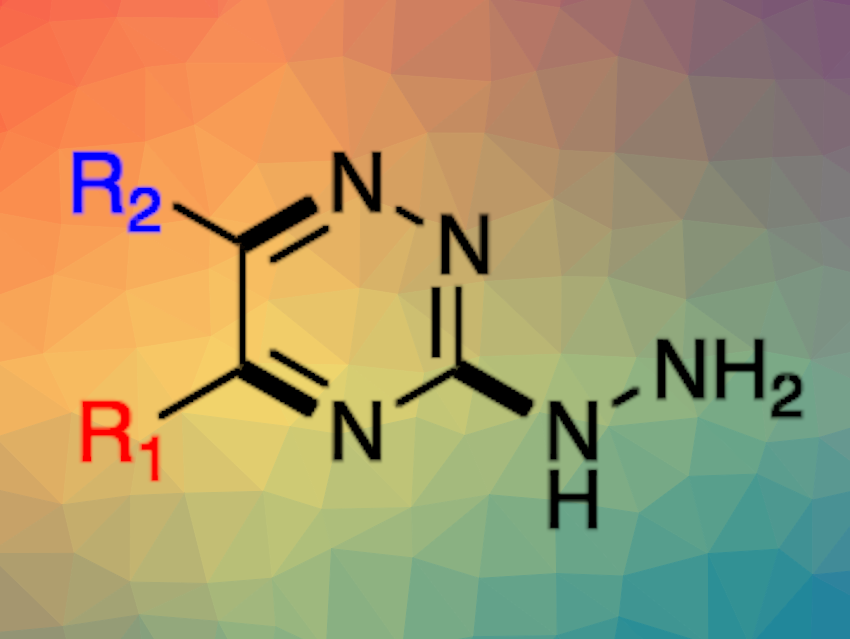
1,2,4-Triazines are six-membered aromatic heterocycles that are found, e.g., in pharmaceutically active compounds. Hydrazone derivatives of 1,2,4-triazines are also interesting in pharmaceutical chemistry. There are various strategies for synthesizing 1,2,4-triazines. However, they can require, for example, multiple steps, harsh reaction conditions, toxic metals or oxidants, excess amounts of acid or base, or long reaction times. In addition, some methods lead to the formation of multiple regioisomers.
Marco Radi, Università degli Studi di Parma, Italy, and colleagues have developed a method for a microwave-assisted, one-pot, three-step, metal-free [4+2] annulation for the synthesis of 5,6-substituted-3-hydrazinyl-1,2,4-triazines (general product structure pictured). The team reacted aromatic or heteroaromatic methyl ketones with I2 in dimethyl sulfoxide (DMSO) to oxidize the α-methyl group, followed by a cyclization with thiosemicarbazide in the presence of K2CO3 and a nucleophilic substitution with hydrazine to obtain the desired product.
Using this approach, the team obtained substituted 5-aryl/heteroaryl-3-hydrazinyl-1,2,4-triazines in moderate to good overall yields. The products can be further transformed, e.g., by reactions with aldehydes/ketones to give a variety of hydrazone derivatives. Overall, the work provides a synthetic strategy for highly substituted 1,2,4-triazines that proceeds under mild conditions and with good functional group compatibility.
- A Microwave‐Assisted One‐Pot Three‐Step Metal‐Free [4+2] Annulation for the Sustainable Synthesis of Highly Substituted 1,2,4‐Triazines,
Daniele Rubini, Maria Grazia Martina, Matteo Incerti, Francesca Barbieri, Marco Radi,
Eur. J. Org. Chem. 2024.
https://doi.org/10.1002/ejoc.202400087