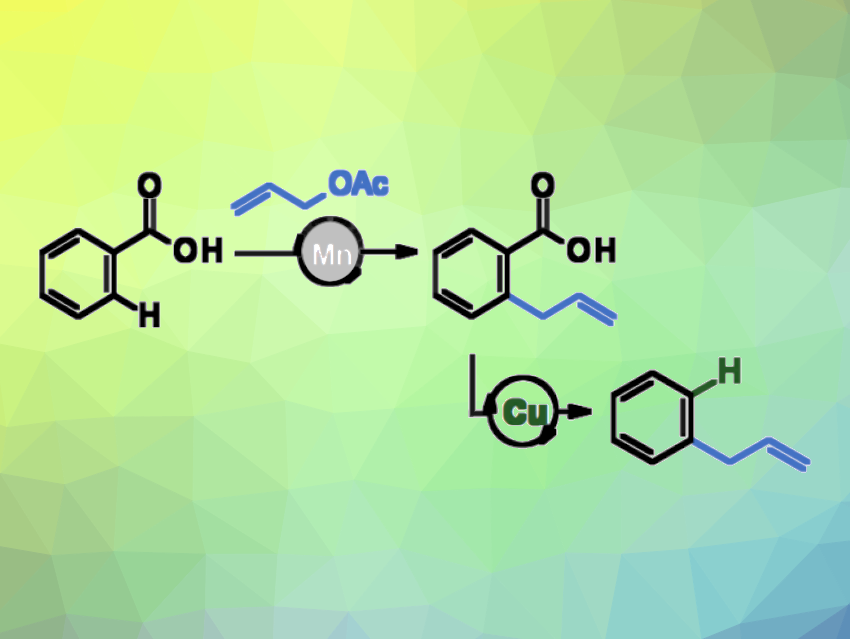
Allylated arenes are useful, e.g., in the flavor and fragrance industry or in pharmaceutical and materials science. Allyl groups can be introduced using cross-coupling reactions with pre-functionalized arene substrates or Friedel-Crafts-type reactions. These approaches can be limited in the accessible substitution patterns. Metal-catalyzed, directed C–H allylations can provide a path to allyl arenes with other substitution patterns. However, the installation and removal of directing groups can be cumbersome. Simple carboxylates can be useful alternative directing groups because benzoic acids are widely available and the carboxylates can be easily removed by decarboxylation.
Lukas J. Gooßen and colleagues, Ruhr University Bochum, Germany, have found that an inexpensive and non-toxic Mn(I) complex can efficiently catalyze the ortho-allylation of a variety of benzoic acids under mild conditions. This reaction can be combined with an in situ decarboxylation, providing access to allylarenes in substitution patterns that are otherwise hard to obtain (reaction sequence pictured). The team used MnBrCO5 as the catalyst together with neocuproine as a ligand and reacted a variety of benzoic acid derivatives with different substituted allyl acetates, using tetrahydrofuran (THF) as the solvent. The reactions were performed at 60 °C.
The desired allyated products were obtained in moderate to good yields. After the allylation, the carboxylate directing group could be removed as part of a one-pot reaction sequence. The team added a solution of copper(I) oxide in N-methyl-2-pyrrolidone (NMP)/quinoline after completion of the allylation step, followed by heating the reaction mixture in the microwave to achieve the desired decarboxylation. This sequence provides convenient access to allylbenzenes with substitution patterns that are traditionally only accessible in multistep syntheses.
- Manganese(I) Catalyzed ortho C–H Allylation of Benzoic Acids,
Jonas Felix Goebel, Johanna Stemmer, Florian Belitz, Lukas J. Gooßen,
Angew. Chem. Int. Ed. 2023.
https://doi.org/10.1002/anie.202301839