Supercritical fluids, i.e., substances at a temperature and pressure above their critical points, have interesting and often useful properties. The critical point is the point in the phase diagram where the liquid–vapor boundary ends—for water, for example, the critical temperature is ca. 647 K and the critical pressure is ca. 22 MPa. Confining a substance within a porous material could change these physicochemical properties.
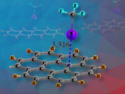
Andrea Le Donne, University of Ferrara, Italy, Paweł Zajdel, Mirosław Chorążewski, University of Silesia, Chorzów, Poland, Yaroslav Grosu, University of Silesia, Katowice, Poland, and Centre for Cooperative Research on Alternative Energies (CIC energiGUNE), Basque Research and Technology Alliance (BRTA), Vitoria-Gasteiz, Spain, and colleagues have found that the supercritical transition for water confined within a specific metal–organic framework (MOF) takes place at a much lower temperature than in bulk water. The team used the hydrophobic MOF Cu2tebpz (tebpz = 3,3′,5,5′-tetraethyl-4,4′-bipyrazolate) and performed high-pressure intrusion–extrusion experiments with water.
At ambient pressure, the pores of the MOF can be considered to be occupied by water in its confined vapor phase. The researchers measured the volume of an overall sample containing the MOF and water at different pressures for a given temperature. They observed sudden volume changes at certain pressures due to the intrusion of liquid water into the material. At temperatures over ca. 400 K, this “jump” in volume disappeared. This can be attributed to achieving supercriticality: When the confined fluid becomes supercritical, the difference between the densities of liquid-like and vapor-like confined water disappears, and so does the discontinuity in the volume change.
This observation corresponds to an unprecedented reduction in critical temperature of ca. 200–250 K. The team proposes that this reduction is due to the large density of the vapor-like phase, which is supported by synchrotron measurements that show the formation of water clusters in the MOF. They point out that the work could lead to new applications of hydrophobic porous materials.
- Mild-Temperature Supercritical Water Confined in Hydrophobic Metal–Organic Frameworks,
Sebastiano Merchiori, Andrea Le Donne, Josh D. Littlefair, Alexander Rowland Lowe, Jiang-Jing Yu, Xu-Dong Wu, Mian Li, Dan Li, Monika Geppert-Rybczyńska, Lukasz Scheller, Benjamin A. Trump, Andrey A. Yakovenko, Paweł Zajdel, Mirosław Chorążewski, Yaroslav Grosu, Simone Meloni,
J. Am. Chem. Soc. 2024.
https://doi.org/10.1021/jacs.4c01226