Halogen∙∙∙arene interactions are interesting research targets and have been observed in many crystal structures. Investigating this type of interaction in solution can be challenging, in part due to the large variance in the steric demands of the halogens. The large differences in the radii of halogen atoms can prevent systematic studies using supramolecular complexes or molecular balances that include the larger halogens.
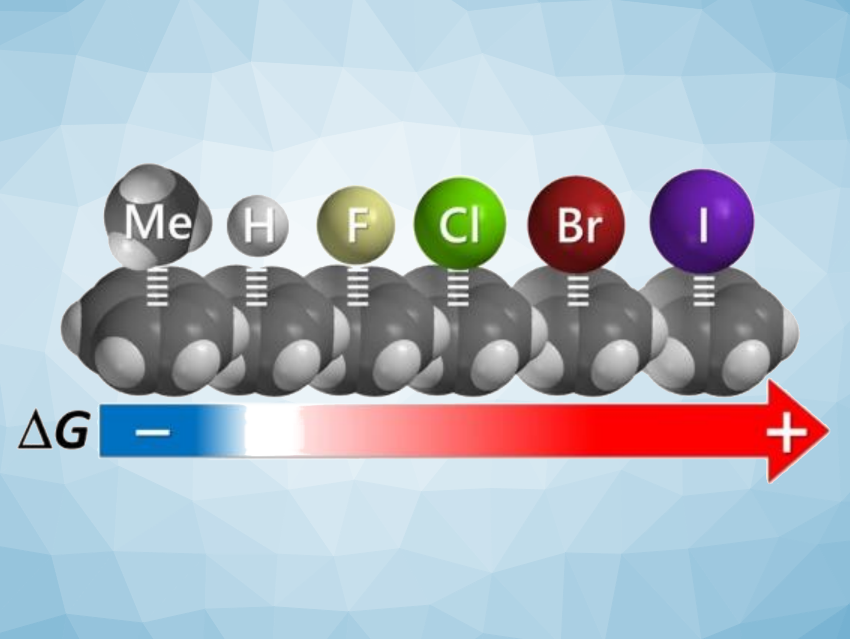
Scott Lee Cockroft, University of Edinburgh, UK, and colleagues have adapted so-called Wilcox molecular torsion balances, which are usually employed to study CH-π interactions, to examine halogen∙∙∙arene contacts (pictured below). In these molecular balances, iodo, bromo, chloro, fluoro, or methyl substituents can be consistently brought into contact with the face of the terminal phenyl ring in the folded conformation.
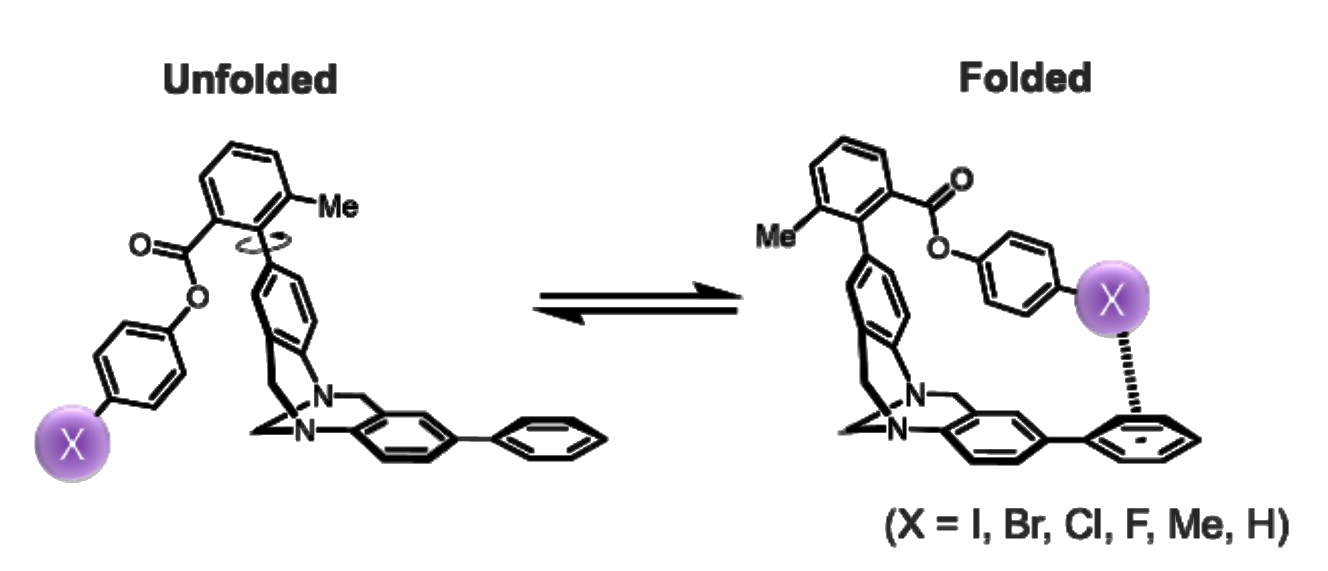
This approach allowed the team to quantify the effects of halogen∙∙∙arene contacts on the equilibrium between the folded and unfolded conformations in 17 solvents and solvent mixtures using 1H NMR spectroscopy. The experimental results were compared with gas-phase computational data.
The team found that side-on halogen∙∙∙arene contacts were weakly disfavored in all examined solvents. Comparable methyl∙∙∙arene interactions were weakly favorable in all cases. Computational results supported the hypothesis that the methyl∙∙∙arene interactions are favored over the halogen∙∙∙arene interactions due to electrostatic attraction, which is greatly diminished in halogen∙∙∙arene interactions. Overall, the work indicates that dispersion interactions alone are insufficient to drive the association of halogen∙∙∙arene contacts in solution, even between functional groups considered to be highly polarizable.
- Experimental Quantification of Halogen∙∙∙Arene van der Waals Contacts,
Andrew Mark London West, Nicholas Dominelli-Whiteley, Ivan V. Smolyar, Gary S. Nichol, Scott Lee Cockroft,
Angew. Chem. Int. Ed. 2023.
https://doi.org/10.1002/anie.202309682