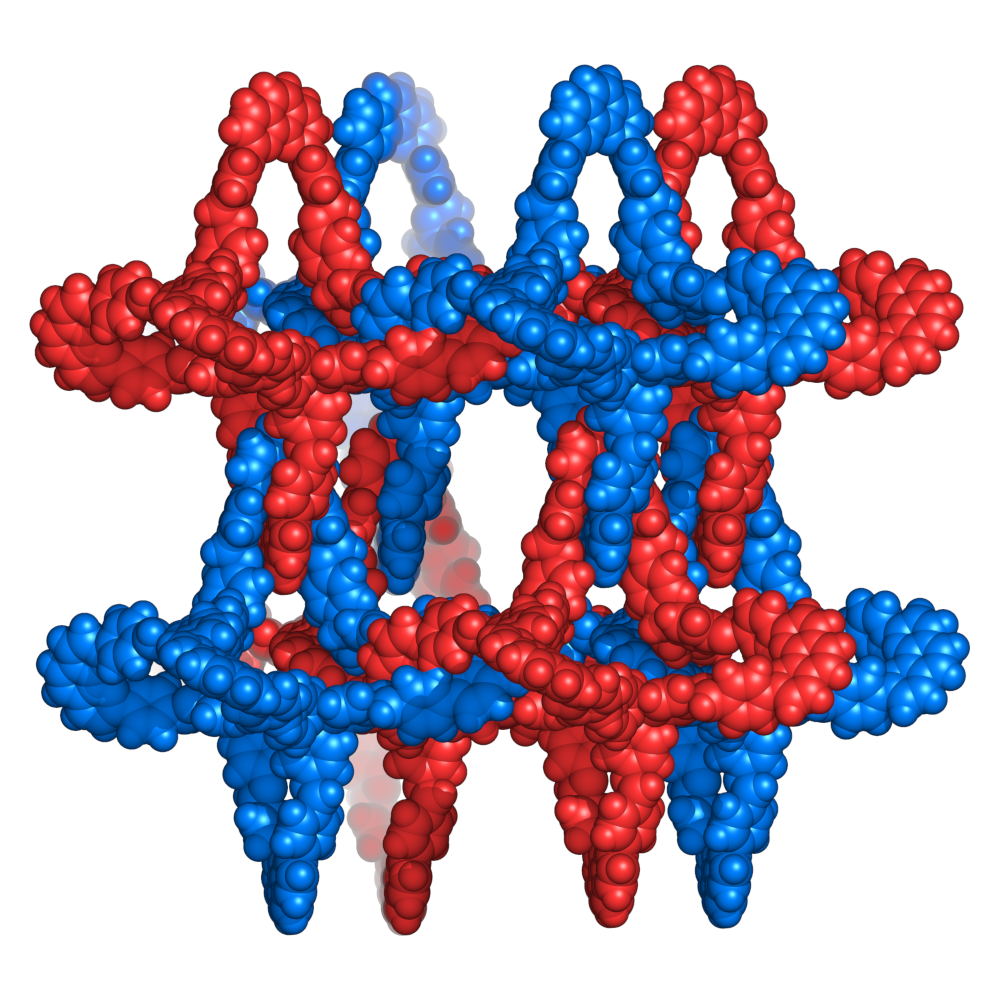
Catenanes are mechanically-interlocked structures with two or more interlocking rings that have potential, e.g, as molecular machines. However, building molecular structures with very large numbers of interlocking rings, as for example in a chainmail-like structure, is a challenge. To achieve this, many rings must interlock in a precise, concerted fashion, and in an ordered arrangement. A potentially useful approach to tackling this challenge is reticular chemistry. In reticular chemistry, instead of building chemical structures one molecule at a time, extended structures such as metal–organic frameworks (MOFs) and covalent organic frameworks (COFs) are generated in a one-step reaction using molecular building blocks.
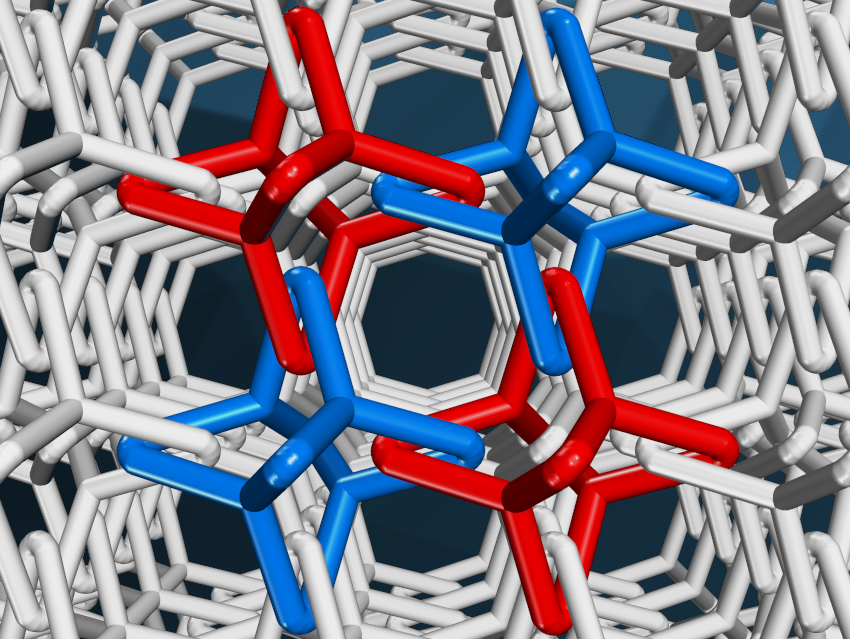
Omar M. Yaghi, University of California, Berkeley, and Kavli Energy NanoScience Institute, Berkeley, CA, USA, and colleagues have used reticular chemistry to create 3D chainmail structures composed of millions of interlocking polyhedra (simplified structure pictured above). They call these catenated covalent organic frameworks “infinite” catenanes ([∞]catenanes). In the product, each polyhedron is interlocked with six others, creating an extended 3D chainmail.
Catena-COFs
The team first conceptually dissected the target chainmail structure into its most fundamental units. Molecular building blocks with the desired shapes were then linked together using established imine reactions. The team linked Cu(I)-bis[4,4′-(1,10-phenanthroline-2,9-diyl)dibenzaldehyde]tetrafluoroborate ([Cu(PDB)2]BF4) with tris-(4-aminophenyl)amine (TAPA), tris-(4-aminophenyl)methane (TAPM), or tris-(4-aminophenyl)methanol (TAPMol). The copper ions were used to coordinate two PDB units each, with the four aldehyde groups pre-organized in a roughly tetrahedral arrangement. The condensation reactions with the employed amine linkers then gave the desired interlocked, adamantane-like polyhedra (example of a partial structure pictured below, colors used to make the discrete polyhedra easier to see).
The team achieved postsynthetic removal of the copper(I) ions (up to 90 %) by adding aqueous KCN to obtain the corresponding COF. The researchers anticipate that such interlocking structures could be useful in flexible materials that retain their robustness and resilience. This could have applications, e.g., in soft robotics, bullet-proof structures, or materials that require stiffening on demand.
- Catenated covalent organic frameworks constructed from polyhedra,
Tianqiong Ma, Yi Zhou, Christian S. Diercks, Junpyo Kwon, Felipe Gándara, Hao Lyu, Nikita Hanikel, Pilar Pena-Sánchez, Yuzhong Liu, Nicolas J. Diercks, Robert O. Ritchie, Davide M. Proserpio, Osamu Terasaki, Omar M. Yaghi,
Nat. Synth. 2023.
https://doi.org/10.1038/s44160-022-00224-z