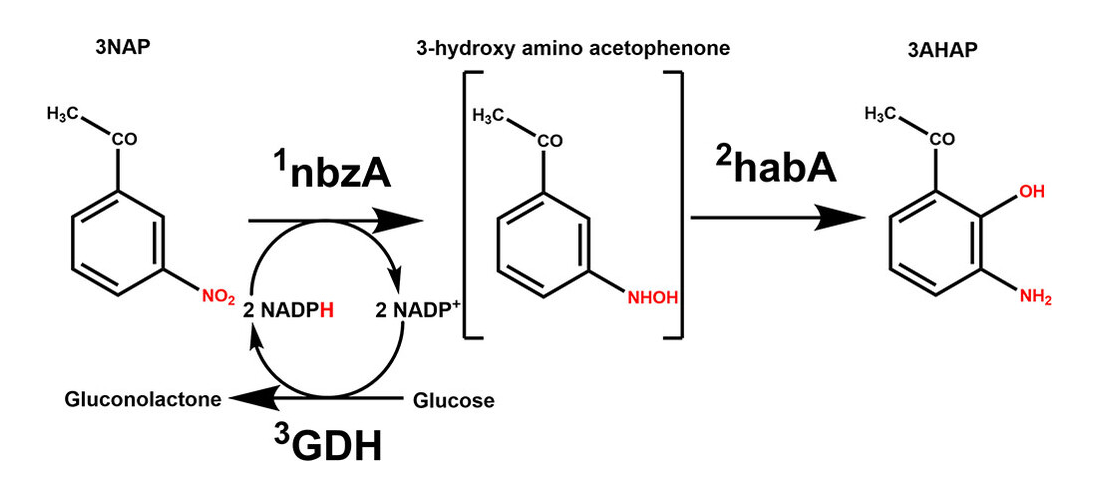
3-Amino-2-hydroxy acetophenone (3AHAP, pictured below) is a critical intermediate in the synthesis of pranlukast, a commonly prescribed asthma medication. Biosynthetic routes to 3AHAP can be more environmentally friendly than chemosynthetic routes. For example, a combination of the enzymes nitrobenzene nitroreductase (nbzA) and hydroxylaminobenzene mutase (habA) can be useful for the synthesis of 3AHAP.
However, this process requires nicotinamide adenine dinucleotide phosphate (NADPH) as a cofactor, which can be expensive. Glucose dehydrogenase (GDH) can be used as a “cofactor regenerator” for NADPH to solve this problem.
Ya-Ping Xue, Zhejiang University of Technology, Hangzhou, China, and colleagues have coupled GDH with nbzA and habA to create a three-enzyme synthetic route for producing 3AHAP from m-nitroacetophenone (3NAP) in vitro. The reaction proceeds via 3-hydroxy amino acetophenone (pictured below). To increase the yield of 3AHAP for industrial production, the team adjusted enzyme molar ratios to enhance the complex reaction system. The highest yield of 3AHAP was achieved at a molar ratio of nbzA/habA/GDH of 1:4:24.
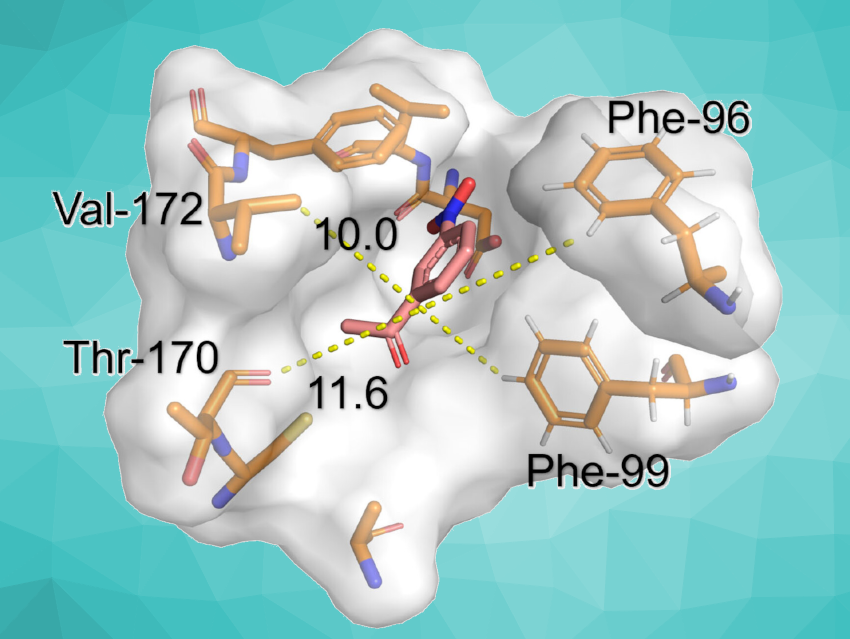
The researchers also used site-directed mutagenesis to enhance the activity of the enzyme habA. The habA mutant had an expanded active pocket volume, increasing from ca. 176 Å3 in the wild type to ca. 209 ų. It also showed a higher affinity for the substrate than the “original” enzyme. After optimization, the yield of 3AHAP was up to 580 mg L–1, the highest reported yield using biosynthesis. The work could have implications for the industrial production of 3AHAP.
- Efficient Production of 3‐Amino‐2‐Hydroxy Acetophenone by Multi‐Enzyme Biosynthesis,
Heng Tang, Hong‐Li Zhu, Jin‐Xing Zhong, Meng‐Nan Wang, Ya‐Ping Xue, Yu‐Guo Zheng,
ChemBioChem 2023.
https://doi.org/10.1002/cbic.202300165