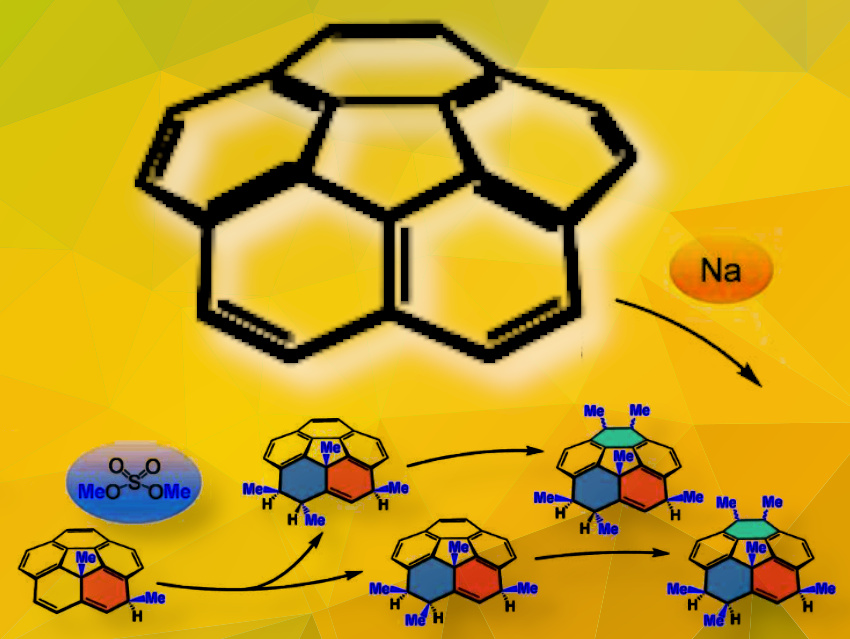
Corannulene (C20H10) is a bowl-shaped polycyclic aromatic hydrocarbon (PAH) with the partial structure of C60 fullerene. A variety of corannulene-based molecules with unique structures and properties have been created so far by peripheral substitution reactions on the rim carbon atoms. While surface addition reactions via anionic corannulene species are also known, their potential remains largely unexplored, as they have been limited only to hydrogenation.
Shinobu Aoyagi, Nagoya City University, Takahiro Sasamori, University of Tsukuba, Hideki Yorimitsu, Kyoto University, all Japan, and colleagues, have synthesize multiply exo-methylated corannulenes using a reductive surface functionalization method. The team treated corannulene with excess amounts of sodium dispersion and dimethyl sulfate in tetrahydrofuran. The treatment led to the in-situ iterative reduction/methylation sequences that involves the reduction of corannulene using sodium to form anionic corannulene species, followed by SN2 reactions of these anionic species with reduction-resistant dimethyl sulfate. As a result, exo-di-, tetra-, and hexamethylated corannulenes were generated (pictured above).
To confirm the molecular structures of the multi-methylated corannulenes and the sequence of the multi-methylation process, the team used various analytical techniques including X-ray diffraction analyses, NMR, MS, UV-Vis measurements, as well as DFT calculations.
The reductive surface functionalization method can be applied to multiple surface functionalizations of other curved PAHs to create unprecedented intriguing molecules. By using appropriate “reduction-resistant” electrophiles instead of dimethyl sulfate, a variety of functional groups other than methyl groups can be efficiently introduced on the curved surface.
- Multiply exo-Methylated Corannulenes,
Kazuhira Miwa, Shinobu Aoyagi, Toru Amaya, Takahiro Sasamori, Shogo Morisako, Takashi Kurogi, Hideki Yorimitsu,
Chem. Eur. J. 2023.
https://doi.org/10.1002/chem.202301557


![A Path to Substituted Bicyclo[2.1.1]hexanones](https://www.chemistryviews.org/wp-content/uploads/2024/10/1substitutedbicyclo211hexan2ones_2024-125x94.png)
