Alkenylboronate esters are important organic building blocks used for cross-coupling reactions like the Suzuki–Miyaura reaction. There are approaches for the synthesis of alkenylboronic acid derivatives that require toxic and/or corrosive reagents. Hydroboration reactions of alkynes are useful alternatives to these processes. Improved catalysts for these reactions are an attractive research target.
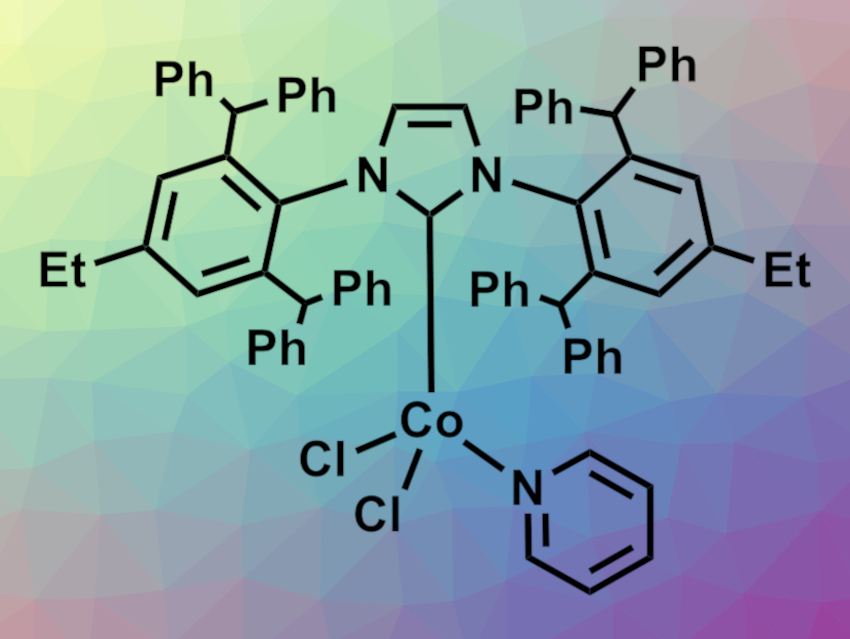
Małgorzata Bołt and Patrycja Żak, Adam Mickiewicz University, Poznań, Poland, have developed a cobalt complex with a bulky N-heterocyclic carbene (NHC) ligand (pictured) as a pre-catalyst for alkyne hydroborations. The complex was prepared from anhydrous cobalt(II) chloride, the carbene, and pyridine.
The team found that the complex can promote the hydroboration of both terminal and internal alkynes, giving anti-Markovnikov products. A variety of alkynes was reacted with pinacolborane using LiHBEt3 as a base under solvent-free conditions at 80 °C. For solid reactants, small amounts of toluene were added.
The desired products were obtained in good to excellent yields. When diynes were used as substrates, mono- or bis-borylated products were obtained depending on the stoichiometry of the reactants. The approach avoids a need for solvents, uses a non-precious metal, and provides high selectivity.
- Solvent-free hydroboration of alkynes catalyzed by an NHC–cobalt complex,
Małgorzata Bołt, Patrycja Żak,
RSC Adv. 2022.
https://doi.org/10.1039/d2ra03005e