The enantioselective synthesis of organic molecules is particularly important in pharmaceutical chemistry because different enantiomers often have different biological effects. Axially chiral biaryl phosphines are useful tools for enantioselective catalysis. Synthesizing analogous compounds containing nitrogen in the axially chiral backbone is challenging due to the easier rotation around the central bond, which can lead to racemization. The enantioselective synthesis of atropisomeric biaryls with sterically demanding substituents is difficult to achieve with available catalysts, but the specific properties of C–N axially chiral ligands could be well-suited for this task.
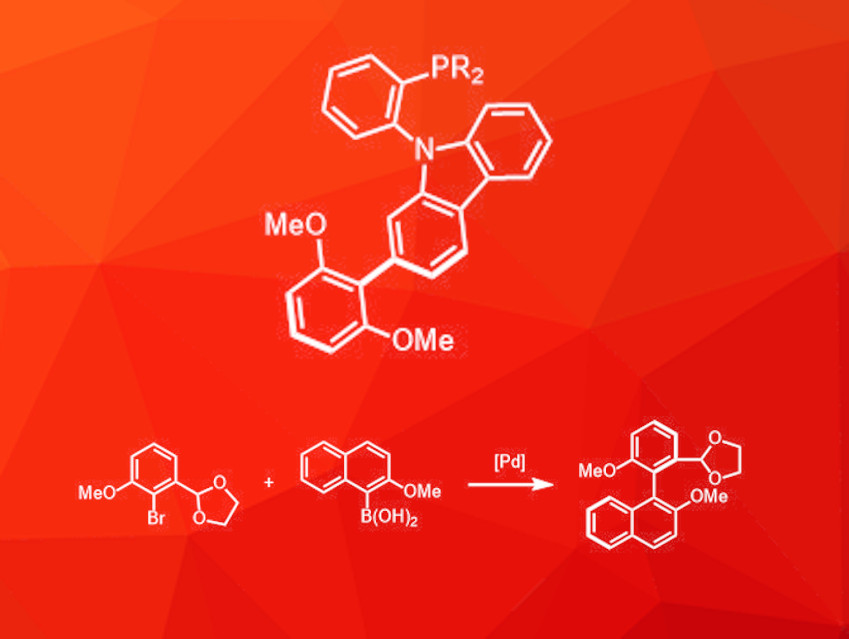
Rong-Lin Zhong, The Chinese University of Hong Kong, Shatin, and Jilin University, Changchun, China, Zhen-Wei Zhang, The Chinese University of Hong Kong and Guangxi University of Chinese Medicine, Nanning, China, Fuk Yee Kwong, The Chinese University of Hong Kong, and colleagues have developed an asymmetric phosphine ligand with an axially chiral 2,6-dimethoxyphenyl-substituted carbazole backbone (pictured). This type of ligand can be used in enantioselective Pd-catalyzed Suzuki–Miyaura cross-coupling reactions that give highly sterically hindered tetra-ortho-substituted biaryls (example pictured).
The ligand system was chosen because it can take part in different interactions: The carbazole unit offers two different coordination modes for the Pd center, either via the nitrogen atom or the π-system, and the methoxy groups can form hydrogen bonds with the substrate and improve chiral induction. The ligand was synthesized in a modular four-step procedure from commercially available building blocks. The half-time for its racemization is about 135 days, i.e., the ligand is sufficiently stable.
The ligand was then used in enantioselective cross-coupling reactions of aryl bromides and organoboron compounds to obtain tetra-ortho-substituted biaryl compounds. Various substrates with both electron-withdrawing and electron-donating substituents are well tolerated and the reaction provides excellent enantiomeric ratios (up to 98:2).
- Atropisomeric Phosphine Ligands Bearing C–N Axial Chirality: Applications in Enantioselective Suzuki–Miyaura Cross-Coupling Towards the Assembly of Tetra-ortho-Substituted Biaryls,
Kin Boon Gan, Rong-Lin Zhong, Zhen-Wei Zhang, Fuk Yee Kwong,
J. Am. Chem. Soc. 2022.
https://doi.org/10.1021/jacs.2c06240