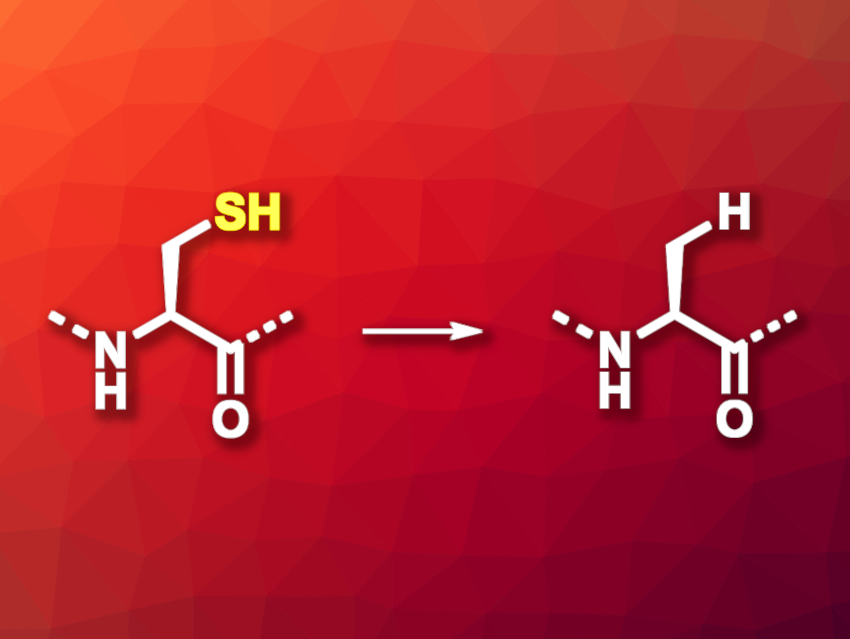
The chemical synthesis of proteins is a useful tool, e.g., in chemical biology. Techniques such as native chemical ligation (NCL) are useful in this context. In NCL, the thiol group of an N-terminal cysteine residue of one peptide reacts with a C-terminal thioester of a second peptide to connect the two peptides. The cysteine remains in the product, which could limit the application of the resulting larger peptide because cysteine is not a very common amino acid in natural proteins. Desulfurization methods that can convert the cysteine residues to the more common alanine (general reaction pictured) are, thus, useful for the further transformation of the products.
Maciej A. Walczak, University of Colorado, Boulder, USA, and colleagues have developed a robust, easy-to-operate desulfurization reaction for peptides and proteins. The team used tris(2-carboxyethyl)phosphine hydrochloride (TCEP), a water-soluble phosphine, as the sole reductant and B2(OH)4 (tetrahydroxydiboron) as a radical initiator for a radical desulfurization. The reactions were performed in a buffer solution containing Na2HPO4 and guanidine at room temperature. According to the team, the diboron reagent produces radicals upon exposure to water and ambient light, so no other radical initiator was needed.
In a model reaction using a pentapeptide with the sequence H-ACFGV–OH, the desired desulfurization product was obtained in a yield of 90 % within 4 h. The team extended the reaction to a variety of other peptides, including peptides with N-terminal or C-terminal cysteine residues, with selenocysteine residues (giving deselenized products), and with multiple cysteine residues. The reaction tolerates commonly used cysteine protecting groups. Larger proteins were also successfully converted.
- Peptide and Protein Desulfurization with Diboron Reagents,
Ruiheng Jing, Maciej A. Walczak,
Org. Lett. 2024.
https://doi.org/10.1021/acs.orglett.4c00609