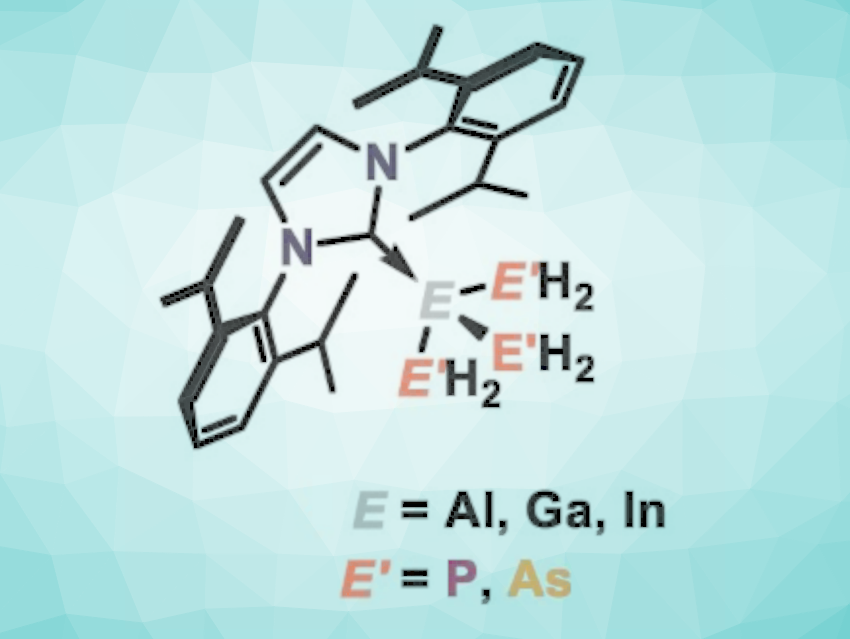
Inorganic equivalents of hydrocarbons can be made up of elements of groups 13 and 15. Such group 13/15 compounds (pentelyltrielanes) are isoelectronic to hydrocarbons, but there are differences, especially due to the strong bond polarization. Therefore, pentelyltrielanes can show intriguing reactivities. They can also be promising precursors for semiconductor applications.
Manfred Scheer, University of Regensburg, Germany, and colleagues have synthesized the elusive parent tripentelyltrielanes (pictured below, E(E’H2)3) stabilized by the bulky N-heterocyclic carbene IDipp (IDipp = 1,3-bis(2,6-diisopropylphenyl)-imidazolin-2-ylidene). By reacting IDipp·ECl3 (E = Ga, Al) with three equivalents of KAsH2 or NaPH2/LiPH2∙DME (DME = dimethoxyethane) at –80 °C under exclusion of air, the team prepared the tripentelyltrielanes IDipp·Ga(PH2)3, IDipp·Ga(AsH2)3, IDipp·Al(PH2)3, and IDipp·Al(AsH2)3. In addition, in similar experiments, the researchers found NMR-spectroscopic evidence of a rare NHC-stabilized triphosphanylindiumane, IDipp·In(PH2)3.
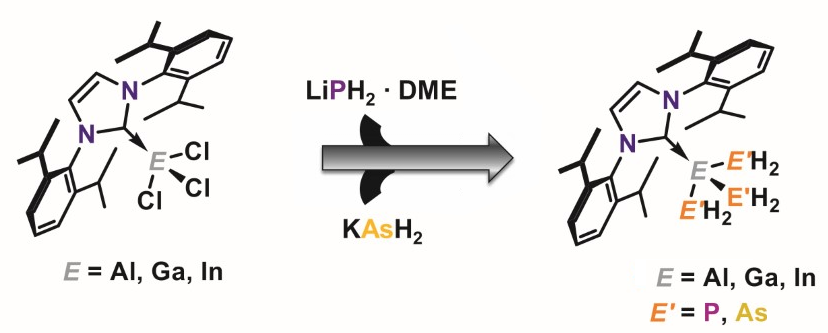
Overall, a method to access a series of novel compounds containing combinations of the heavier group 13/15 elements has been developed. The products display extreme sensitivity towards air and decompose slowly at room temperature. As the compounds contain a well-defined stoichiometry and only bear hydrogen substituents, they might be promising candidates for the deposition of thin group 13/15 layers for semiconductor applications.
- NHC‐Stabilised Parent Tripentelyltrielanes,
Robert Szlosek, Michael Weinhart, Gábor Balázs, Michael Seidl, Lisa Zimmermann, Manfred Scheer,
Chem. Eur. J. 2023.
https://doi.org/10.1002/chem.202300340