Nickel-catalyzed C–H activation reactions have been well-studied. However, enantioselective C(sp3)–H activation with abundant and inexpensive nickel (or other 3d transition metals) is challenging due to the sensitivity of such metals to the structures of the substrates and ligands, as well as a lack of efficient chiral ligands.
Mengchun Ye, Nankai University, Tianjin, China, and colleagues have developed a new type of chiral phosphine oxide ligand for enantioselective nickel-catalyzed C(sp3)–H activation. The team used a partially hydrogenated naphthidine as a chiral backbone to prepare a phosphine oxide, which then ligated nickel and aluminum to form an efficient chiral bimetallic catalyst (H8-BINAPO, pictured below in the blue box).
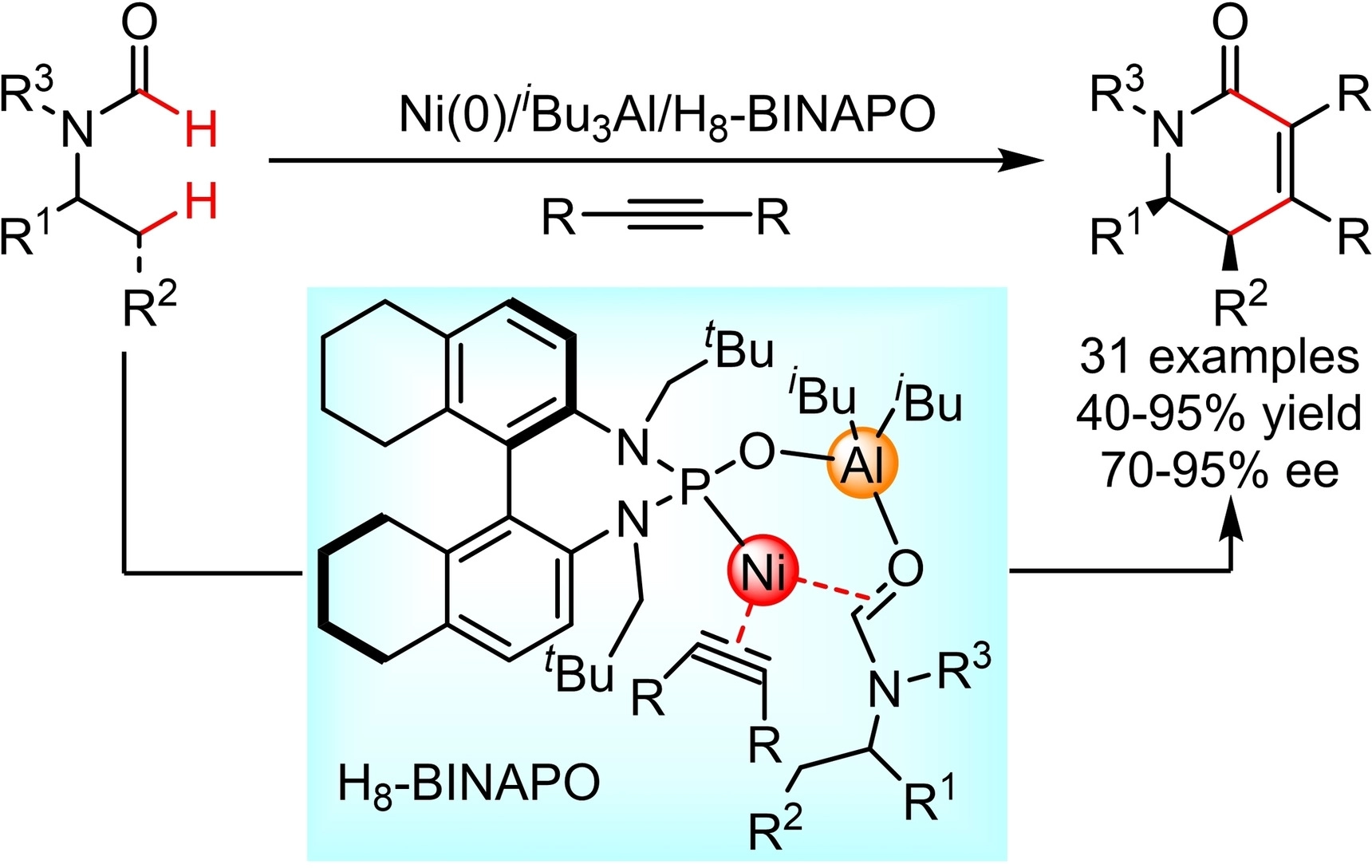
Using this catalyst, the team performed annulations of readily available formamides with alkynes (pictured). They obtained a series of nitrogen-containing heterocycles that are not easily accessible by traditional methods in yields of 40–95 % and with 70–95 % enantiomeric excess. The reaction’s scope includes various diaryl and dialkyl alkynes, as well as primary and secondary C(sp3)–H bonds.
Mechanistic experiments suggest that one formyl C(sp2)–H activation and a rate-determining alkyl C(sp3)–H activation step are involved. According to the researchers, the activity of the bimetallic catalyst may extend to other reactions catalyzed by 3d transition metals.
- Enantioselective Nickel‐Catalyzed C(sp3)−H Activation of Formamides,
Yin‐Xia Wang, Feng‐Ping Zhang, Hao Chen, Yue Li, Jiang‐Fei Li, Mengchun Ye,
Angew. Chem. Int. Ed. 2022.
https://doi.org/10.1002/anie.202209625