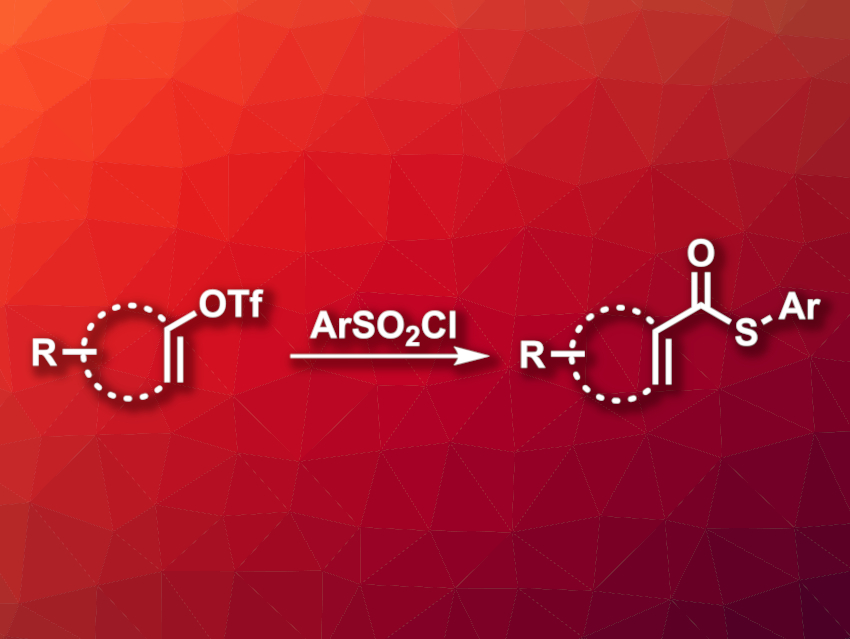
α,β-Unsaturated thioesters are useful building blocks, for example, in the synthesis of complex organic molecules and natural products. They can take part in a wide range of transformations, e.g., in Diels–Alder reactions, cascade cyclizations, decarbonylations, or reduction reactions. Syntheses of α,β-unsaturated thioesters usually rely on condensation reactions. Transition-metal-catalyzed thiocarbonylation reaction of unsaturated compounds provide an alternative to this approach, however, they usually require noble-metal-based catalysts.
Xinxin Qi, Zhejiang Sci-Tech University, Hangzhou, China, Xiao-Feng Wu, Dalian Institute of Chemical Physics, Chinese Academy of Sciences, Liaoning, and Leibniz-Institut für Katalyse e.V., Rostock, Germany, and colleagues have developed a nickel-catalyzed thiocarbonylation of vinyl triflates with arylsulfonyl chlorides (Ar–SO2Cl) to obtain α,β-unsaturated thioesters (pictured). The team used Mo(CO)6 as a CO source, [1,1′-bis(diphenylphosphino)ferrocene]dichloronickel(II) (Ni(dppf)Cl2) as the catalyst, di-tert-butylbipyridine (dtbbpy) as a ligand, Cs2CO3 as a base, water as an additive, and dimethoxyethane (DME) as the solvent. The reactions were performed at 100 °C.
The desired α,β-unsaturated thioesters were obtained in moderate to good yields. The reaction showed good functional group tolerance. According to the researchers, this is the first example of a nickel-catalyzed carbonylative synthesis of α,β-unsaturated thioesters using arylsulfonyl chlorides as the sulfur source.
- Nickel-Catalyzed Carbonylative Synthesis of α,β-Unsaturated Thioesters from Vinyl Triflates and Arylsulfonyl Chlorides,
Yong-Wang Huo, Xinxin Qi, Xiao-Feng Wu,
Org. Lett. 2022.
https://doi.org/10.1021/acs.orglett.2c01430