Cycloarenes, i.e., macrocycles composed of aromatic units, have interesting electronic properties and can be useful, e.g., in organic electronics or sensing. Heteroatoms such as nitrogen, oxygen, or sulfur can be used to tune the properties of such compounds, giving heterocycloarenes. However, synthesizing (hetero)cycloarenes can be challenging, which hampers efforts to tailor their structure and limits their practical application.
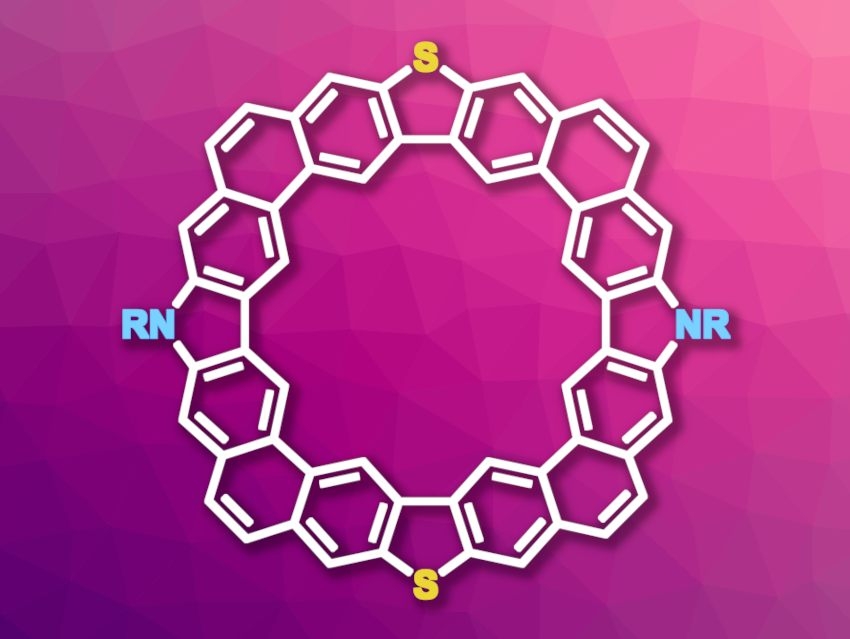
Yan Zhao, Xuefeng Lu, Yunqi Liu, Fudan University, Shanghai, China, and colleagues have synthesized three new nitrogen- and sulfur-codoped cycloarenes (NS-doped octulenes, general structure pictured; R = branched alkyl substituents containing linear spacer groups of length Cn with n = 2, 3, 4). The codoped octulenes contain alternating dibenzo[b,d]thiophene and carbazole units.
The team first synthesized building blocks with the different branched alkyl substituents starting from 2,7-dibromo-9H-carbazole using Hofmann alkylation reactions. The alkylated products were converted to dibrominated dialdehydes and coupled with dibenzo[b,d]thiophene-based building blocks. The resulting macrocycles contain four aldehyde groups, which were converted to methoxyethenyl groups and then used to close the remaining rings via Bi(OTf)3-catalyzed cyclizations.
Analogous undoped octulenes and S-doped octulenes are saddle-shaped, while the NS-doped octulenes are planar. The team found that the NS-doped octulenes can act as hosts in supramolecular chemistry with the fullerenes C60 or C70 as guests. The heteroatoms change the structure and electronic properties of the octulenes and might make them suitable for use, e.g., in organic field-effect transistors (OFETs).
- Alkyl-Substituted N,S-Embedded Heterocycloarenes with a Planar Aromatic Configuration for Hosting Fullerenes and Organic Field-Effect Transistors,
Ning Zhang, Longfei Yang, Wenhao Li, Jiangyu Zhu, Kai Chi, Dongdong Chang, Yanjun Qiao, Teng Wang, Yan Zhao, Xuefeng Lu, Yunqi Liu,
J. Am. Chem. Soc. 2022.
https://doi.org/10.1021/jacs.2c08276