Arylboron compounds are useful intermediates in organic synthesis. The catalytic C–H borylation of arenes is a convenient and atom-economic way to access this type of compound. Usually, transition-metal catalysts are used for this. Methods for the non-metal-catalyzed C–H borylation of arenes could improve the sustainability as well as the regioselectivity and substrate scope. However, there had been no methods for a main-group-catalyzed C–H borylation with unactivated arenes as limiting reagents known so far.
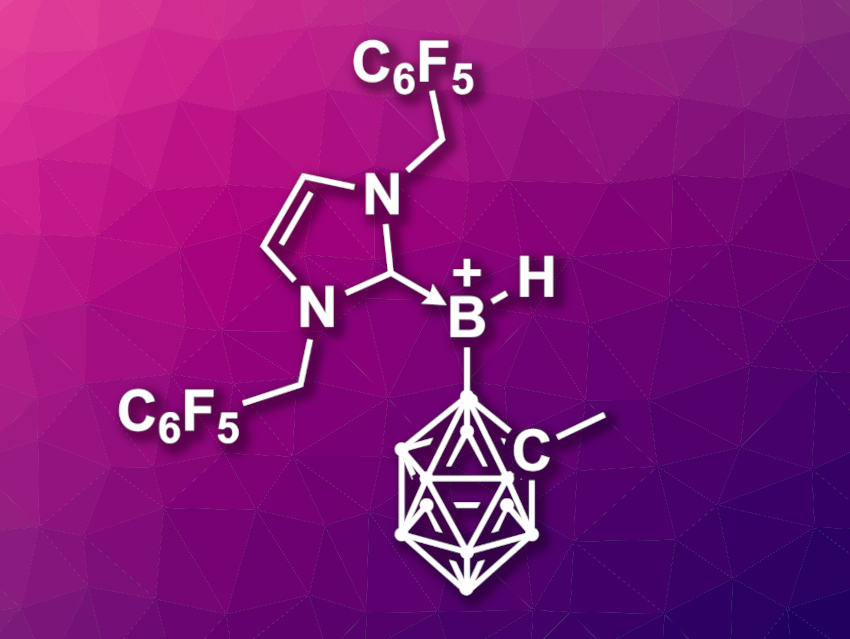
Huadong Wang, Fudan University, Shanghai, China, and colleagues have developed a borenium-ion catalyst (pictured) that can promote the borylation of unactivated arenes using 4-chlorocatecholborane as a borylation reagent under ambient conditions. The catalyst is an N-heterocyclic carbene (NHC)-stabilized o-carboranyl-substituted borenium ion, [IBnFB(H)CbMe]+ (IBnF = 1,3-bis(2,3,4,5,6-pentafluorobenzyl)imidazol-2-ylidene, CbMe = 2-methyl-o-carboran-1-yl). It was prepared in multiple steps starting from 1-Me-o-carborane and IBnFBH3, using [Ph3C][B(C6F5)4] to create the borenium ion.
Using this catalyst, the team reacted unactivated arenes with 4-chlorocatecholborane in o-C6H4F2 as the solvent, followed by a reaction with pinacol and NEt3 to give pinacolatoboron-functionalized arenes. The team found that the catalytic system favors para-selective borylations. The developed approach allows the borylation of C–H bonds in sterically hindered positions and provides high selectivity for the para-borylation of phenols. It can also be used, for example, in a one-pot C–H borylation/Suzuki–Miyaura cross-coupling sequence.
- Borenium-Ion-Catalyzed C–H Borylation of Arenes,
Xinyue Tan, Xi Wang, Zhen Hua Li, Huadong Wang,
J. Am. Chem. Soc. 2022.
https://doi.org/10.1021/jacs.2c12151