Organic luminescent compounds usually contain “classic” chromophores such as π-conjugated aromatic units, which feature aromatic through-bond conjugation (TBC). However, there are small molecules, macromolecules, and supramolecules without any aromatic structures that show intrinsic fluorescence or phosphorescence. These nontraditional luminogens (NTLs) contain nonconventional chromophores such as heteroatoms with electron lone pairs and/or unsaturated bonds. The photoluminescence mechanism of NTLs is generally explained by intra- or intermolecular through-space conjugation (TSC), involving overlap of the “electron clouds” of the nonconventional chromophores.
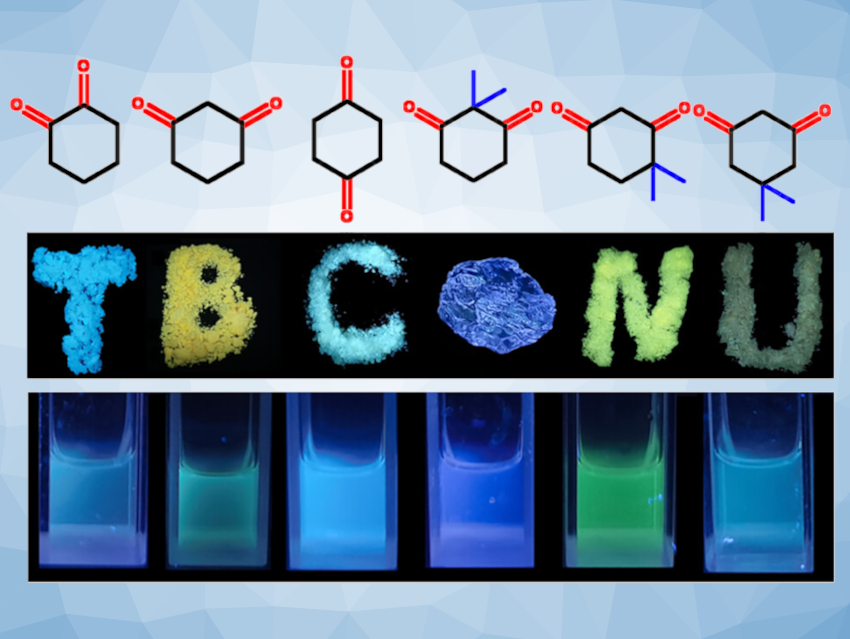
Huiliang Wang, Beijing Normal University, China, and colleagues have proposed differentiating between “classic” aromatic through-bond conjugation and “nonaromatic TBC” as a new concept. They showed that nonaromatic TBC can play an important role in nontraditional luminogens. The team studied the photoluminescence behaviors of three isomers of cyclohexanedione and three isomers of gem-dimethyl-1,3-cyclohexanedione (pictured).
These compounds show different fluorescence emissions from blue to yellow (pictured), which vary with the position of the carbonyl and the methyl groups. Some of the isomers can undergo keto-enol tautomerism, leading to nonaromatic TBC involving the C=O, C=C, and ─OH units. Quantum-chemical calculations showed that the TBC reduces the energy gap between the HOMO (highest occupied molecular orbital) and the LUMO (lowest unoccupied molecular orbital), leading to red-shifted emissions.
This work shows that nonaromatic TBC can play an important role in the photoluminescence of NTLs. This effect, together with through-space conjugation and the aromatic TBC of traditional luminogens, could explain the photoluminescence of organic luminescent materials.
- Effects of nonaromatic through‐bond conjugation and through‐space conjugation on the photoluminescence of nontraditional luminogens,
Xiaomi Zhang, Yunhao Bai, Junwen Deng, Peifeng Zhuang, Huiliang Wang,
Aggregate 2024.
https://doi.org/10.1002/agt2.517