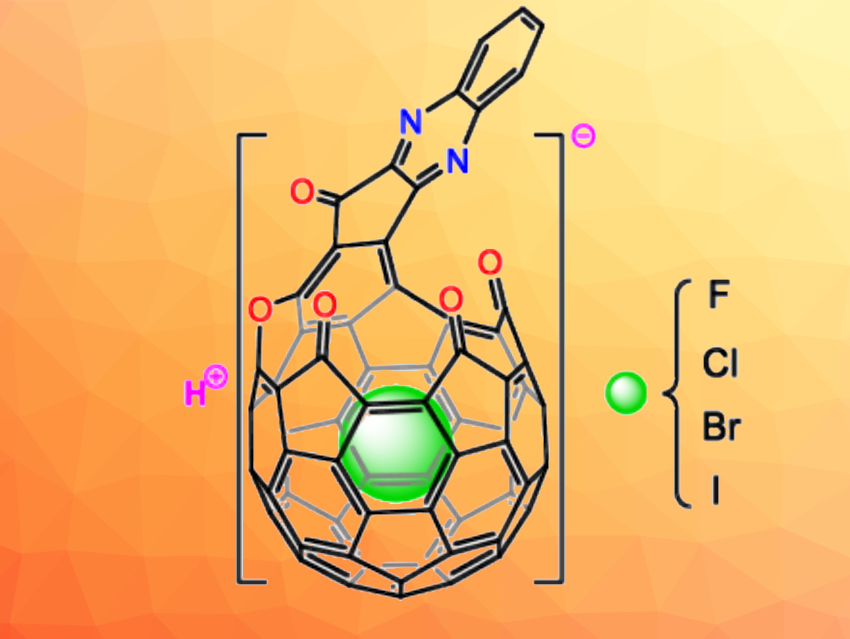
Fullerenes are spherical molecules composed of covalently bonded carbon atoms. Selective cleavage of the bonds in an area on the surface of fullerenes can create an opening and gives an “open-cage fullerene”. Depending on the size of the opening, open-cage fullerenes can function as molecular containers for various guests, including noble gas atoms and small neutral molecules. However, no examples of encapsulated halide anions as guest species had been reported so far.
Yuming Yu, Xinjiang University, China, Jie Su, Liangbing Gan, Peking University, Beijing, China, and colleagues have synthesized a new open-cage C60 derivative A, which can encapsulate halide anions to form H[X@A] (pictured, X = F–, Cl–, Br–, I–). The open-cage fullerene derivative was prepared from C60 in seven steps and 5.5 % yield. There are four carbonyl groups, an ether oxygen, and a quinoxaline group on the rim of the opening.
Chloride anions could be inserted into the open-cage fullerene’s cavity by heating it with hydrochloric acid at 60 °C for 4 h. The encapsulation of fluoride, bromide, or iodide anions using NaF, HBr, or NaI was achieved at 90 °C and in ca. 14 h. In the open-cage fullerene, the positive nature of the cavity and the negative oxygen atoms on the rim of the opening provide the driving force for encapsulation by interacting with the halide anion and the proton, respectively.
- Open‐cage Fullerene as Molecular Container for F–, Cl–, Br– and I–,
Shijun Sun, Zhen Liu, Francesca Colombo, Rui Gao, Yuming Yu, Yi Qiu, Jie Su, Liangbing Gan,
Angew. Chem. Int. Ed. 2022.
https://doi.org/10.1002/anie.202212090