Helicenes are a fasinating class of chiral polycyclic aromatic hydrocarbons (PAHs) consisting of ortho-fused aromatic rings. Of great interest are their potential applications in chiral bioimaging and optoelectronic devices, for which strong near-infrared (NIR) chiroptical activity is highly desirable. However, near-infrared chiroptical response has been less explored because it is challenging to achieve both chirality and NIR absorption/emission.
Hua Lu, Hangzhou Normal University, Xiaoye Wang, Nankai University, China, and colleagues have presented a facile synthesis toward β-isoindigo-based double π-helix BODIPY analogs (β-IBs), with the aim of creating efficient NIR chiroptical molecular materials and investigating the chiral variation.
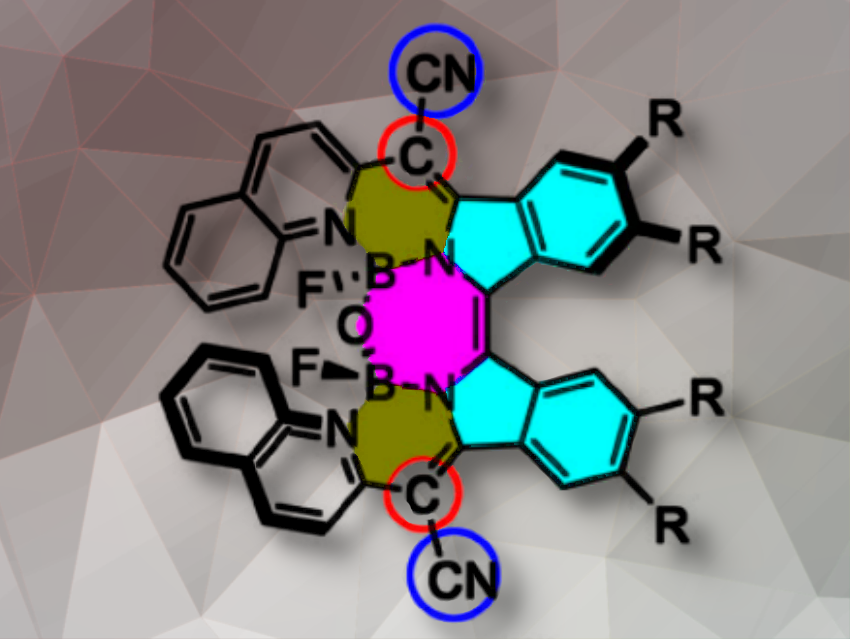
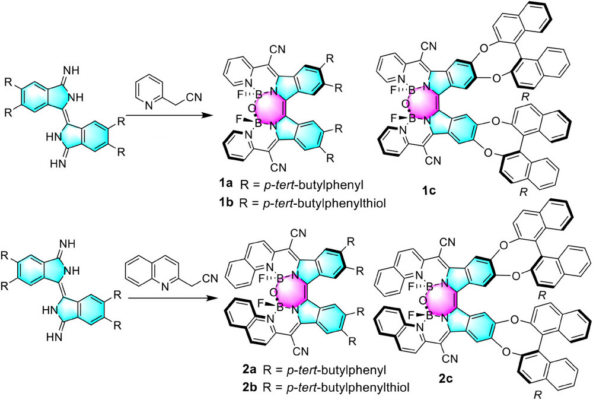
The team prepared the diimino-β-isoindigo derivatives from a 4,5-disubstituted phthalonitrile (pictured below). The diimino-β-isoindigo was reacted with 2-(pyridin-2-yl)acetonitrile or 2-(quinolin-2-yl)acetonitrile to afford the ligand and subsequently coordinated with BF3·OEt2. Spontaneous hydrolysis of adjacent B–F bonds in the constrained cavity by a tiny amount of water produces the B–O–B bridges thus forming highly distorted structures.
The analogs show exceptional chiroptical responses, as evidenced by circular dichroism (CD) spectra across the visible and NIR region, with Cotton effects of 127.8 M-1cm-1 and absorbance dissymmetry factors (|gabs|) of 3.5×10-3. Luminescence dissymmetry factor (glum) and circularly polarized luminescence (CPL) brightness (BCPL) of up to 1.24×10-3 and 1.78 M-1cm-1, respectively, were realized beyond 800 nm. These β-IBs are the first examples of helicene-type compounds with the highest gabs in the NIR region and CPL beyond 800 nm. These impressive properties highlight the potential application of β-isoindigo-based π-helixes as chiral bioimaging and photodetector materials. Theoretical calculations show that the strong chiroptical activities are triggered by their large transition magnetic dipole moments.
According to the researchers, this study provides a simple synthetic approach to the synthesis of a larger variety of unprecedented helicene-type BODIPY analogs and offers a comprehensive insight into the management of NIR chiroptical characteristics, serving as a catalyst for further exploration in the design of NIR-CPL materials.
- Helicene-type β-isoindigo-based boron-dipyrromethene analogs with strong near-infrared chiroptical activity,
Ziwei Chen, Zhigang Ni, Xing-Yu Chen, Yongqiang Xu, Chunyan Yu, SiSi Wang, Xiao-Ye Wang, Hua Lu,
Aggregate 2024.
https://doi.org/10.1002/agt2.498