The activation of carbon monoxide is an interesting research target. It can be achieved using transition metals, but there are also some main-group compounds that can activate CO. For example, some B−B multiply bonded species can react with CO. However, this type of reaction has been limited to symmetrically substituted derivatives, and more than one CO has been required to cleave the B–B bond completely. The insertion of a single CO molecule into B–B multiple bonds had not been observed so far.
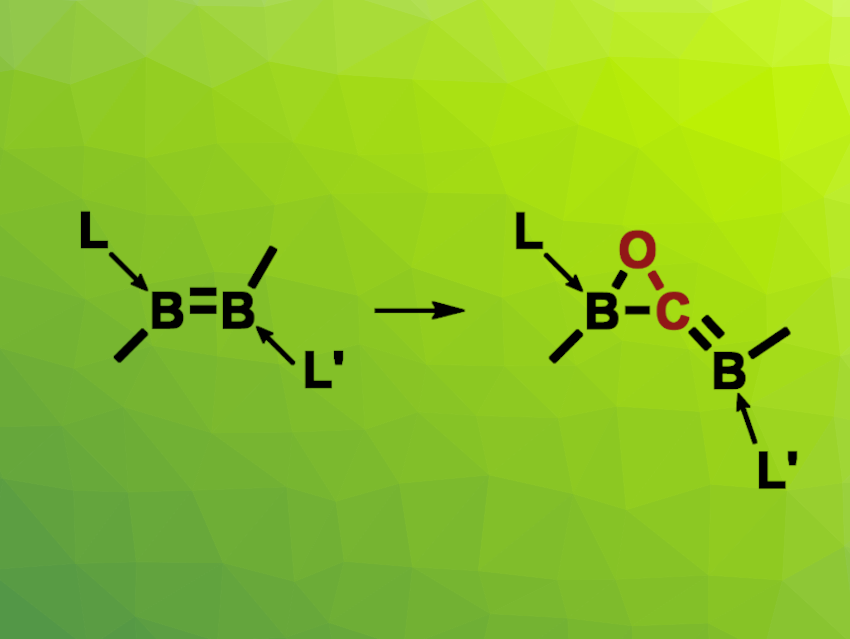
Lizhao Zhua and Rei Kinjo, Nanyang Technological University, Singapore, have observed the insertion of CO into an unsymmetrical diborene to give an oxaborirane species, i.e., a compound with a BCO three-membered ring (pictured). In this reaction, there is a complete scission of the unsymmetrically substituted B![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) B bond upon the insertion of a single CO molecule. The team reacted a diborene with an N-heterocyclic carbene (NHC) ligand and a cyclic (alkyl)(amino)carbene (cAAC) ligand in a toluene solution with a CO atmosphere at room temperature.
B bond upon the insertion of a single CO molecule. The team reacted a diborene with an N-heterocyclic carbene (NHC) ligand and a cyclic (alkyl)(amino)carbene (cAAC) ligand in a toluene solution with a CO atmosphere at room temperature.
The researchers obtained an oxaborirane product in the form of yellow crystals in a yield of 77 %. The product does not react further with CO. Its structure was confirmed via single-crystal X-ray diffraction. Based on density functional theory (DFT) calculations, the team proposes a reaction mechanism that involves the coordination of CO to the boron atom adjacent to the NHC ligand, which leads to an intermediate in which the CO unit is bound between the two boron atoms. This is followed by the formation of a B–O bond to give the oxaborirane.
The team also observed the insertion of isocyanides into unsymmetrical diborenes. This provides access to BCN-type three-membered rings, i.e., azaboriridines.
- Insertion of carbon monoxide into an unsymmetrical diborene to form an oxaborirane,
Lizhao Zhu, Rei Kinjo,
Chem. Commun. 2023.
https://doi.org/10.1039/D3CC02844E