Studying the chemistry of actinides such as plutonium can require anhydrous, halide-based starting materials. However, existing methods for the synthesis of such starting materials can have drawbacks such as a need for rigorous drying methods or strongly oxidizing halogenating reagents. The development of alternative approaches could make further investigations of plutonium chemistry easier.
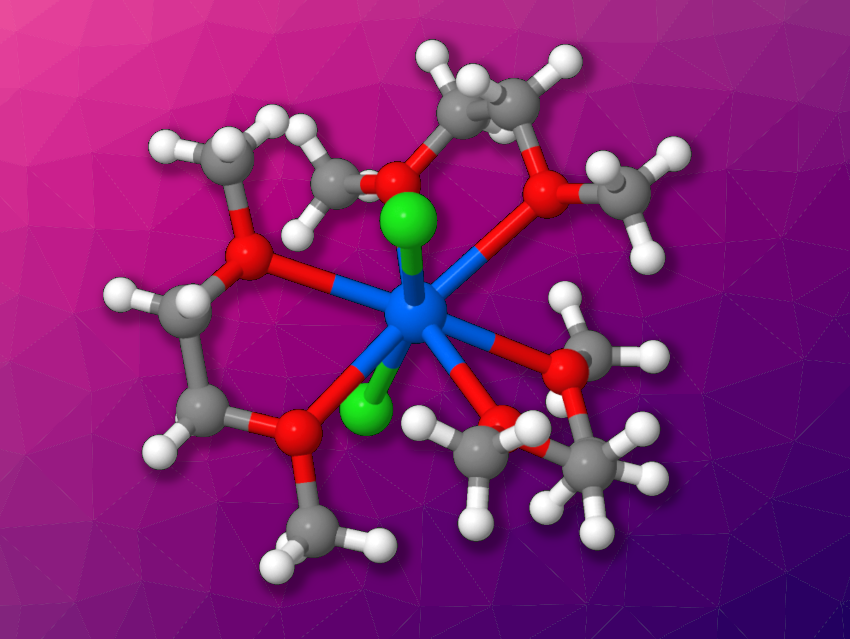
Nickolas H. Anderson, Aaron M. Tondreau, Los Alamos National Laboratory, NM, USA, and colleagues have found that gallium trichloride (GaCl3) can be used for the oxidative chlorination of plutonium metal, as well as uranium metal, to generate coordination complexes. The researchers reacted plutonium or uranium pieces with GaCl3 in 1,2-dimethoxyethane (DME) at room temperature over several days. For plutonium, 60 % of the metal was converted in ten days, and the team obtained [PuCl2(dme)3][GaCl4] (cation pictured above). The product is an eight-coordinate plutonium complex with three equatorial DME ligands and two axial chloride ligands.
With uranium, the researchers obtained [UCl(dme)3][GaCl4]2. The structure of this compound features highly disordered DME ligands. When the team attempted to recrystallize this product, they obtained the bridged complex [{U(dme)3}2(μ-Cl3)][GaCl4]3. The generated products have potential uses as synthetic precursors for plutonium and uranium chemistry.
- Chlorination of Pu and U Metal Using GaCl3,
Stephanie H. Carpenter, Bonnie E. Klamm, Taylor V. Fetrow, Brian L. Scott, Andrew J. Gaunt, Nickolas H. Anderson, Aaron M. Tondreau,
Inorg. Chem. 2023.
https://doi.org/10.1021/acs.inorgchem.3c00522