Bismuth is a nontoxic main-group metal, and bismuth compounds can be used in organic synthesis, e.g., as Lewis acid catalysts or oxidants. Nevertheless, bismuth compounds have rarely been used as redox catalysts. The development of new reactions that use bismuth compounds as catalysts would, thus, be useful.
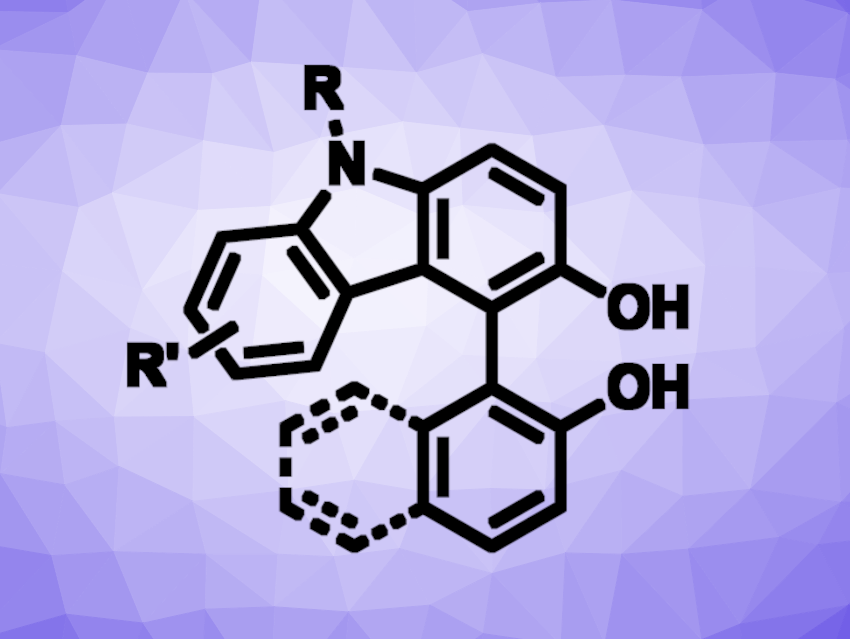
Kengo Kasama, Takayuki Yakura, University of Toyama, Japan, and colleagues have developed a method for the Bi(III)-catalyzed oxidative cross-coupling reaction of 3-hydroxycarbazoles with arenols. The reaction proceeds under mild conditions and gives hydroxybiaryls (general product structure pictured). The team used Bi(OTf)3 as the catalyst, MeCN as the solvent, and molecular oxygen as a terminal oxidant and reacted different 3-hydroxycarbazoles with a range of 2-naphthols or phenols at 30 °C.
The desired hydroxybiaryls were obtained in mostly good yields (up to 94 %). The protocol is atom-efficient, has a broad substrate scope, and runs smoothly at low temperatures. According to the researchers, the reaction mechanism is still unknown, and further studies are ongoing.
- Bismuth(III)-Catalyzed Oxidative Cross-Coupling of 3-Hydroxycarbazoles with Arenols under an Oxygen Atmosphere,
Kengo Kasama, Yuta Koike, Haoyang Dai, Takayuki Yakura,
Org. Lett. 2023.
https://doi.org/10.1021/acs.orglett.3c02211