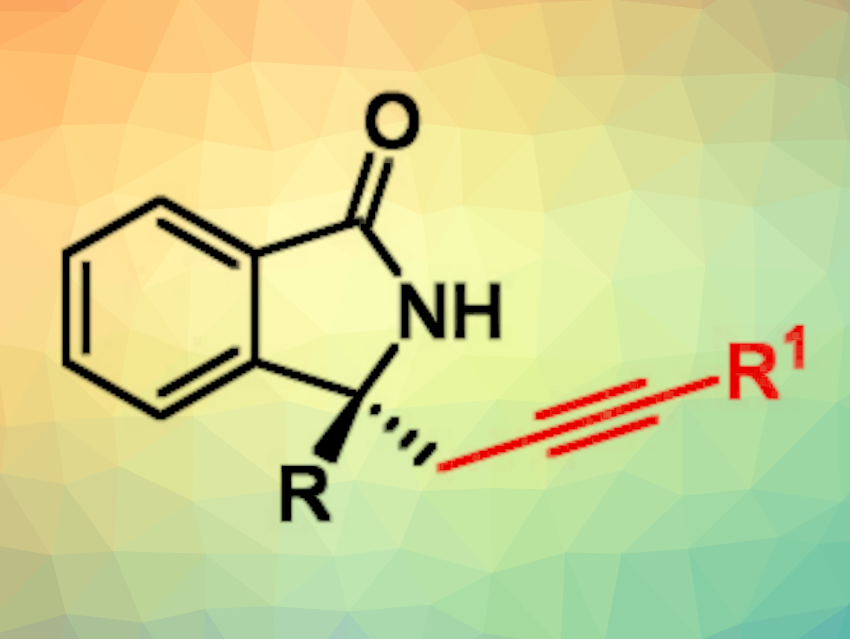
Isoindolines are bicyclic molecules consisting of a benzene ring fused to a five-membered ring containing a nitrogen atom. Derivatives with a ketone group—i.e., isoindolinones—and a tetrasubstituted carbon stereocenter are sometimes found in bioactive natural products and can be interesting for drug development. Efficient methods for the asymmetric synthesis of chiral isoindolinones are, thus, interesting research targets.
Lei Wang, Ying Han, Yangzhou University, China, Chaoshen Zhang, The Hong Kong University of Science and Technology, Kowloon, Hong Kong SAR, China, Junliang Zhang, Yangzhou University and Fudan University, Shanghai, China, and colleagues have developed a method for the asymmetric palladium-catalyzed aminoalkynylation of O-phenyl hydroxamic ethers using terminal alkynes. This reaction gives chiral isoindolinones (simplified structure pictured).
The team used a variety of O-phenyl hydroxamic ethers and terminal alkynes as substrates and reacted them in the presence of [Pd(dmba)Cl]2 (dmba = N,N-dimethylbenzylamine) as a catalyst together with a sterically hindered chiral phosphine ligand, and KOtBu as a base. The reactions were performed in ethyl acetate at 50 °C.
The desired alkyne-substituted chiral isoindolinones were obtained in mostly moderate to good yields and with high enantioselectivities. The reaction was performed on a gram scale, and the products can be further functionalized, e.g., using the alkyne group.
- Enantioselective Synthesis of Isoindolinone by Palladium-Catalyzed Aminoalkynylation of O-Phenyl Hydroxamic Ethers with Alkynes,
Lei Wang, Yinqiang Wang, Shuaijie Wu, Chao-Guo Yan, Chaoshen Zhang, Junliang Zhang, Ying Han,
J. Am. Chem. Soc. 2024.
https://doi.org/10.1021/jacs.3c12996