Arynes (i.e. benzyne derivatives) are highly reactive strained alkynes. They have to be generated in situ due to their instability, but can be very useful for different transformations. Often, aryne precursors require harsh reaction conditions or highly reactive reagents for the generation of the corresponding arynes. In contrast, ortho-(trimethylsilyl)phenyl triflates, or Kobayashi aryne precursors, can generate arynes under mild conditions upon addition of a fluoride source. They can be used, for example, in the synthesis of polycyclic aromatic hydrocarbons (PAHs) using palladium-catalyzed co-cyclizations to give triphenylenes.
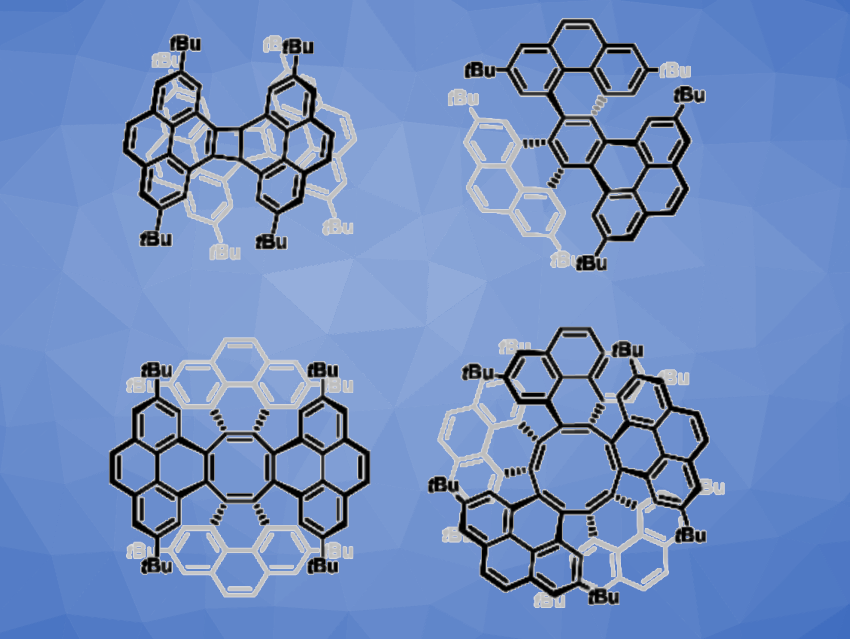
Michael Mastalerz, University of Heidelberg, Germany, and colleagues have found that this type of reaction can also be used to give higher homologues with up to ten-membered central rings (pyrenylenes, examples pictured above). The team prepared a suitable di-tert-butyl-functionalized ortho-(trimethylsilyl)phenyl triflate precursor starting from 4-Bpin-di-tert-butyl pyrene. This precursor was treated with CsF in the presence of Pd2dba3 (tris(dibenzylideneacetone)dipalladium) at room temperature, using MeCN as the solvent.
Using MALDI-TOF mass spectrometry (MALDI = matrix-assisted laser desorption/ionization, TOF = time of flight) to investigate the crude reaction mixture, the team found that dimers to hexamers were formed from the precursor. Using methods such as column chromatography, precipitation, recycling HPLC (HPLC = high-performance liquid chromatography), and recycling GPC (GPC = gel permeation chromatography), the researchers separated the products. The structures were confirmed by single-crystal X-ray diffraction.
- Palladium‐Catalyzed Cyclization of a Pyryne Precursor to Higher Pyrenylenes,
Dennis Popp, Sven M. Elbert, Chantal Barwig, Julian Petry, Frank Rominger, Michael Mastalerz,
Angew. Chem. Int. Ed. 2023.
https://doi.org/10.1002/anie.202219277