Sulfur-containing heterocycles can be useful, e.g., in the development of pharmaceuticals, agrochemicals, and organic electronic materials. Five- and six-membered sulfur-containing heterocycles are most common, but larger, “medium-sized” rings are also often found, for example, in drugs. 1,3-Dithianes, i.e., six-membered rings with two sulfur atoms can undergo ring expansion and serve as precursors for medium-sized heterocycles with two sulfur atoms.
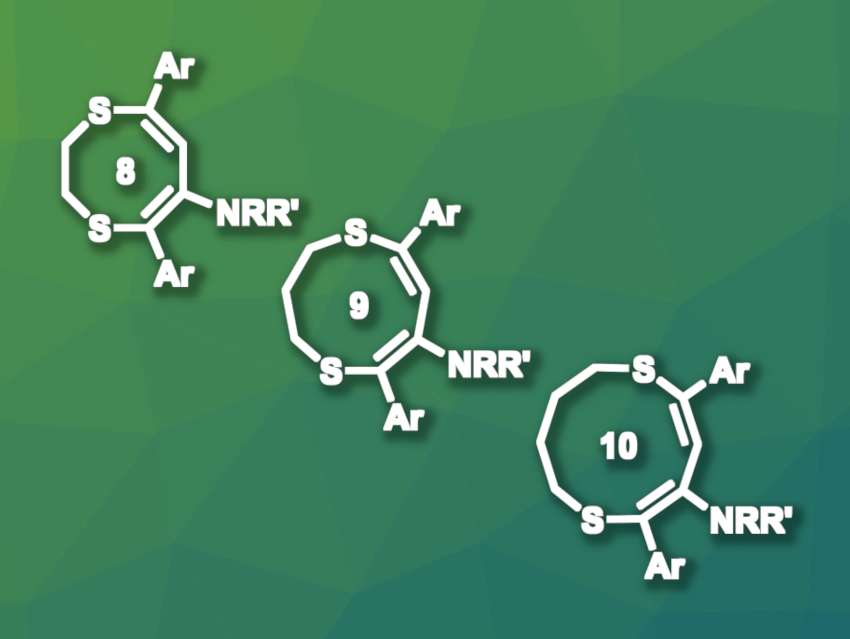
Baris Yucel, Istanbul Technical University, Türkiye, and colleagues have developed a base-mediated rearrangement of dithianyl-substituted propargylamines via expansion of the dithiane ring, using dimethylformamide (DMF) as the solvent. 1,3-Dithianyl-substituted propargylamines were heated with KOtBu as a base in DMF at 40 °C for 4 h in the presence of water and produced the corresponding nine-membered S,S-heterocycles (general structure pictured) in moderate to high yields. This worked with aryl or heteroaryl substituents on the dithiane ring, while a methyl group in this position led to eight-membered rings with an exocyclic double bond.
When the team used substrates based on five-membered dithiolane rings and seven-membered dithiepane rings, they obtained eight-membered and ten-membered products, respectively (general structures pictured). The researchers propose that the reaction takes place via an endo-dig radical cyclization process that starts with the deprotonation of DMF, followed by the formation of a carbamoyl radical. This radical then abstracts the propargylic hydrogen and initiates the desired cyclization. Overall, the work provides access to amino-functionalized S,S-heterocycles of various ring sizes.
- Base-Mediated Rearrangement of α-Dithioacetyl Propargylamines via Expansion of Dithioacetyl Ring: Synthesis of Medium-Sized S,S-Heterocycles,
Mert Dinc, Eda Ismailoglu, Zeynep Mert, Kerem Kaya, Melda Tayanc, Baris Yucel,
Org. Lett. 2023.
https://doi.org/10.1021/acs.orglett.3c01118