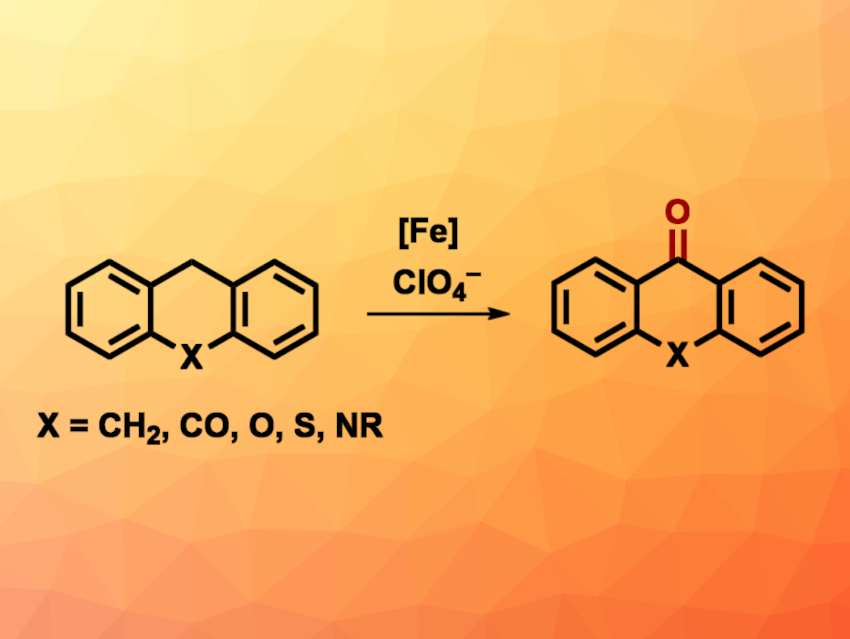
Perchlorate (ClO4–) is a strong oxidant that can also be found as a pollutant in groundwater due to its use in, e.g., pyrotechnics and munitions. Reusing perchlorate in chemical synthesis can be challenging, however, due to its relative kinetic inertness. Thus, suitable catalysts for this are interesting research targets.
Nathaniel K. Szymczak, University of Michigan, Ann Arbor, USA, and colleagues have developed a method for the iron-catalyzed C–H oxygenation of organic substrates using perchlorate ions (general reaction pictured). The team used iron(II) complexes with tris(2-pyridylmethyl)amine (TPA)-type ligands featuring aniline-based H-bond donors as catalysts to promote the reduction of the perchlorate ions. The team optimized the reaction conditions for the oxidation of anthracene and used a ligand with tert-butyl groups at the aniline unit, [NBu4][ClO4] as the chlorate source, AgOTf to capture the formed chloride ions, and EtCN as the solvent. The reactions were performed at 120 °C under a nitrogen atmosphere.
Under these conditions, different cyclic alkyl aromatics and anthracene derivatives were converted to the corresponding quinones/ketones in yields of up to 98 %. According to the researchers, work to expand the scope of the reaction is ongoing.
- Iron-Catalyzed C–H Oxygenation Using Perchlorate Enabled by Secondary Sphere Hydrogen Bonds,
Writhabrata Sarkar, Andrew LaDuca, Jessica R. Wilson, Nathaniel K. Szymczak,
J. Am. Chem. Soc. 2024.
https://doi.org/10.1021/jacs.3c14433