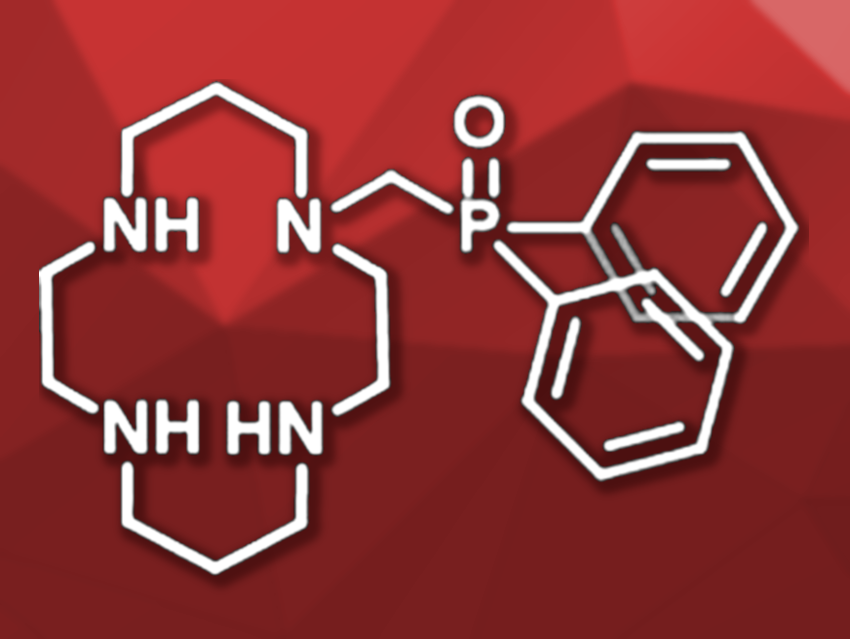
Ligands based on the macrocyclic compound 1,4,8,11-tetraazatetradecane (i.e., cyclams) can form strong complexes with Cu2+, but also with other divalent metal cations such as Zn2+, Ni2+, or Co2+. Copper chelate complexes can have applications, e.g., in medical imaging, where the selective binding of one specific metal is preferable. For Cu2+, five or six coordinating atoms are most suitable—thus, cyclam ligands with one or two additional donor groups could provide selective binding of Cu2+.
Thibault Troadec, Raphaël Tripier, University of Brest, France, and colleagues have developed cyclam ligands with additional phosphine oxide substituents. The free cyclam was selectively protected by tert-butoxycarbonyl groups and reacted with paraformaldehyde and diphenyl phosphine oxide in acetonitrile at 80 °C. The protected ligands were obtained in 95 % yield for a mono(diphenylphosphine oxide)-functionalized derivative and in 58 % yield for a disubstituted derivative. After deprotection, the desired ligands were isolated as hydrochloride salts.
The monosubstituted ligand is soluble in water. At physiological pH, its Cu2+ complex was found to be stable for at least 30 hours. In UV/Vis studies, the characteristic absorption band of the copper complex at 579 nm was visible. In the presence of Zn2+, Co2+, or Ni2+, no complexation takes place and the absorption of the free ligand is observed. The simultaneous presence of Cu2+ and an excess of the competing cations also selectively led to the copper(II) complex. These results confirm that the developed mono(diphenylphosphine oxide)-functionalized ligand shows copper(II) specificity. According to the researchers, it is the first member of the cyclam family to show this behavior.
- A Phosphine Oxide-Functionalized Cyclam as a Specific Copper(II) Chelator,
Marie M. Le Roy, Simon Héry, Nathalie Saffon-Merceron, Carlos Platas-Iglesias, Thibault Troadec, Raphaël Tripier,
Inorg. Chem. 2023.
https://doi.org/10.1021/acs.inorgchem.3c00329