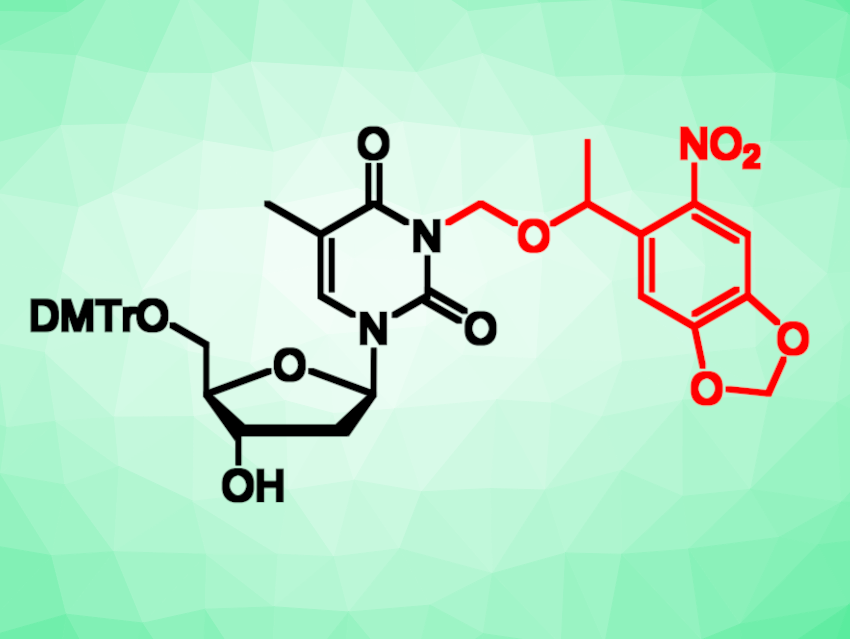
Caging groups are photolabile protecting groups that render a molecule biologically inactive until they are removed through irradiation with light of an appropriate wavelength. They are used to control biological processes with temporal and spatial precision. The 6-nitropiperonyloxymethylene (NPOM) group, for example, has been used as a caging group, most commonly to regulate the function of nucleic acids. The NPOM caging group is traditionally activated under UV light, but the absorbance spectra of NPOM-caged substrates usually tail into the visible range.
Alexander Deiters and Anirban Bardhan, University of Pittsburgh, PA, USA, have investigated the photochemical properties of NPOM under near-visible light using a caged thymidine as the substrate (pictured, DMTr = dimethoxytrityl). The team started from commercially available 5′-O-dimethoxytrityl thymidine, which was reacted with NPOM chloride to install the caging group. They irradiated the resulting caged thymidine at three different wavelengths in the near-visible range (405, 415, and 447 nm) and used high-performance liquid chromatography (HPLC) to detect the amount of caged and decaged thymidine after irradiation.
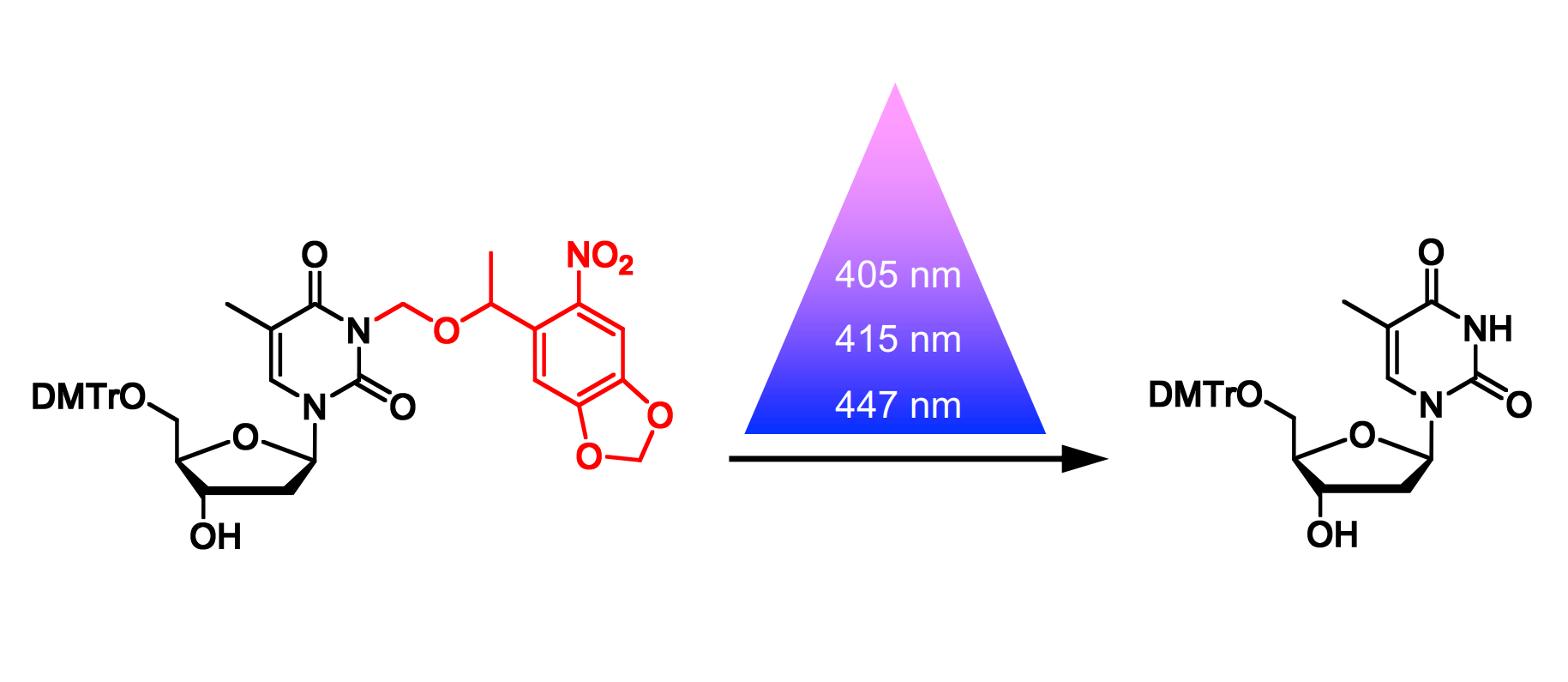
The team found that NPOM is a highly efficient caging group and sensitive to rapid photolysis at wavelengths of 405 nm and 415 nm (within 2 min and 5 min, respectively). This enables the photolysis of NPOM-caged substrates, e.g., with standard imaging lasers on confocal microscopes. The sensitivity of NPOM-cages substrates to blue light may also allow for sequential photolysis when used in combination with a second type of caging group.
- Efficient Activation of 6‐Nitropiperonyloxymethylene (NPOM)‐Caged Nucleosides with Visible Light,
Anirban Bardhan, Alexander Deiters,
ChemPhotoChem 2022.
https://doi.org/10.1002/cptc.202200218