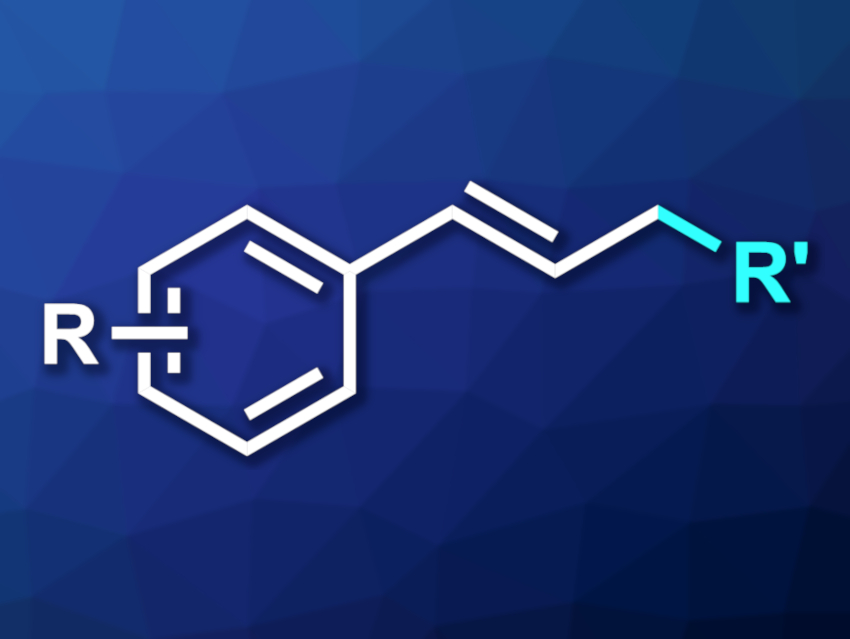
Reactions that form C–C bonds are very useful tools in organic synthesis. Transition-metal-catalyzed allylic alkylations are examples of this type of reaction. Existing approaches to this transformation often require nucleophilic coupling partners that are generated in situ under harsh conditions. Radical reactions with nickel catalysis or dual nickel- and photocatalysis can be promising alternatives. Light-mediated hydrogen atom transfer (HAT) reactions can be useful to break the C–H bonds in alkanes, create radicals, and allow further reactions. Tetrabutylammonium decatungstate (TBADT). for example, is an effective HAT photocatalyst.
Hajime Hirao, The Chinese University of Hong Kong, Shenzhen, Guangdong, Liu-Zhu Gong, University of Science and Technology of China, Hefei, Anhui, and colleagues have developed a method for the allylic alkylation of alkanes under mild conditions, combining nickel catalysis and a decatungstate HAT photocatalyst. The team reacted different phenylallyl carbonates with alkanes in the presence of NiBr2·dtbbpy (dtbbpy = 4,4′-di-tert-butyl-2,2′-bipyridine) as the nickel catalyst and TBADT as the photocatalyst, using acetonitrile as the solvent and LEDs as a light source. The reactions were performed at room temperature.
The desired products were obtained in generally moderate to good yields. The team proposes a mechanism that involves activation of TBADT by light, followed by abstraction of a hydrogen atom from the alkane to form a radical. This radical then takes part in the nickel-catalyzed cycle. In this cycle, the nickel catalyst forms a complex with the allyl substrate, the carbonate in the substrate is displaced, and the nickel is reduced by a tungsten species. This is followed by a reductive elimination, radical addition, and decomplexation to give the desired product.
- Photochemical Allylation of Alkanes Enabled by Nickel Catalysis,
Youxiang Jin, Elvis Wang Hei Ng, Tao Fan, Hajime Hirao, Liu-Zhu Gong,
ACS Catal. 2022.
https://doi.org/10.1021/acscatal.2c02345