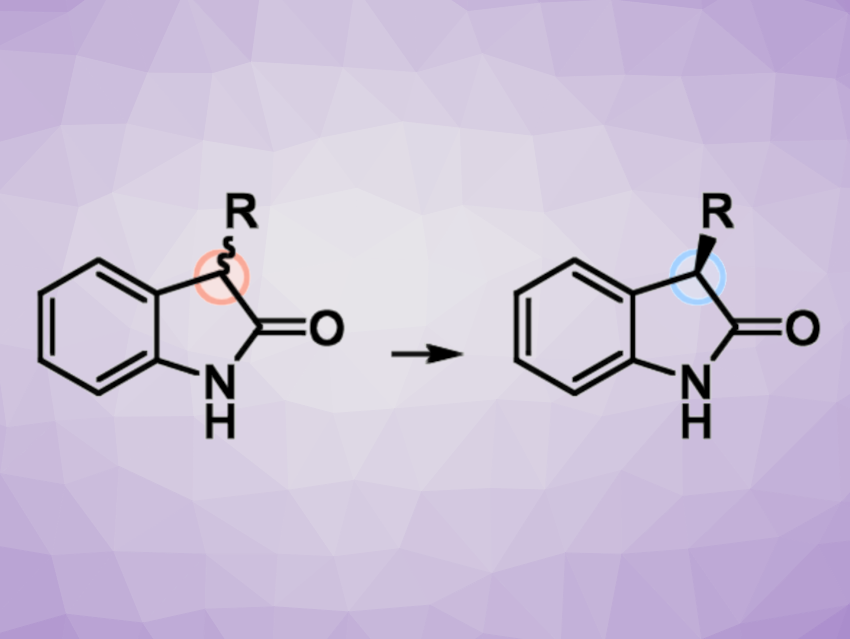
2-Indolinones (oxindoles) make up an important class of heterocyclic compounds with a wide range of biological activities. Many oxindoles are chiral, with one or two substituents at the C3 position. In contrast to 3,3-disubstituted oxindoles, 3-monosubstituted derivatives can be prone to racemization. This can hamper their enantioselective synthesis. One approach to solving this problem could be the use of photochemical deracemization under mild conditions to prepare 3-substituted oxindoles in enantiomerically enriched form from a racemic mixture.
Thorsten Bach, Technische Universität München, Garching, Germany, and colleagues have converted such racemic 3-substituted oxindoles into enantiomerically pure or enriched material (example reaction pictured) using a chiral benzophenone catalyst. The benzophenone underwent excitation under fluorescent lamps with an emission maximum at 366 nm. The team chose a catalyst concentration of 10 mol% and a substrate concentration of 5 mM. Trifluorotoluene was used as the solvent, with an optimal reaction time of 9 h.
The desired products were obtained with high enantiomeric excess (up to 99% ee). Deracemations were performed, e.g., for 3-arylmethyl substituted oxindoles with various substituents or analogous pyridine derivatives. Aliphatic substituents were also tolerated, as well as substitution of the aromatic core. Very bulky groups such as adamantyl close to the C3 carbon atom prevent the reaction. According to the researchers, the transformation likely occurs via a reversible hydrogen atom transfer (HAT) in a substrate-catalyst complex. The enantiomerically pure or enriched oxindole products can serve as useful substrates for further reactions.
- Photochemical Deracemization of 3‐Substituted Oxindoles,
Johannes Großkopf, Alexandra A. Heidecker, Thorsten Bach,
Angew. Chem. Int. Ed. 2023.
https://doi.org/10.1002/anie.202305274