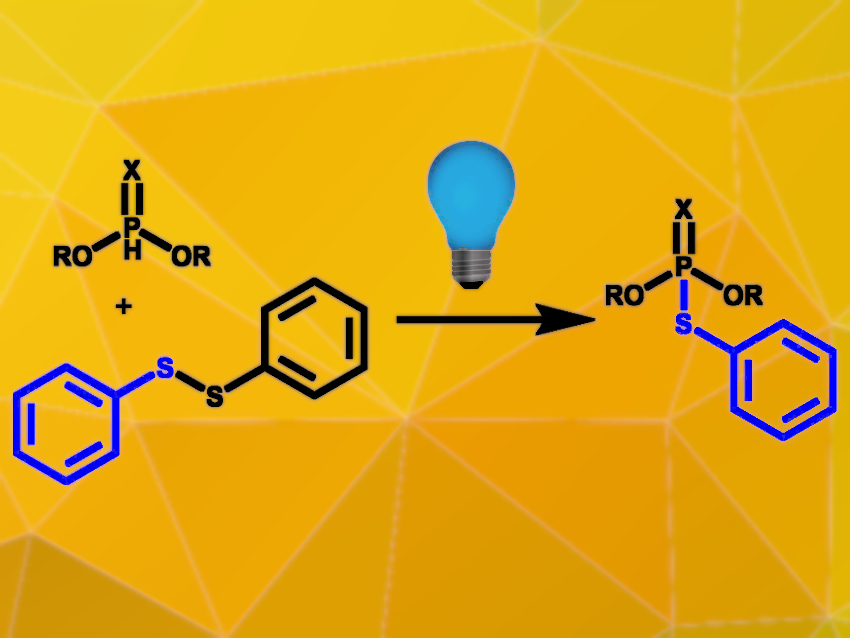
Phosphorothioates and phosphorodithioates (sulfur analogues of phosphates) are often biologically active and can be used, e.g., in pharmaceuticals or pesticides. Additionally, they are important synthetic intermediates for the construction of more complex molecules. Therefore, efficient syntheses of phosphorothioates are useful. Established synthesis routes often depend on expensive metal catalysts or borylated intermediates which generate large amounts of waste. Synthesis methods with fewer waste products are desirable for both economic efficiency and environmental safety.
Chin-Fa Lee, National Chung Hsing University, Taichung City, Taiwan, have developed a metal- and photocatalyst-free photochemical synthesis route for phosphorothioates and phosphorodithioates (example pictured). Alkyl phosphites or phosphonothioates and organic disulfides were coupled in the presence of triethylamine under blue LED irradiation in ethyl acetate. The reaction was carried out at room temperature under ambient air over 16 hours.
A wide range of functional groups on both the phosphorus component and the disulfide component are tolerated, and good to excellent yields are achieved. Alkyl-substituted disulfides react to the desired products but are less active than the aryl equivalents. The reaction also proceeds well with diselenides or ditellurides instead of disulfides. The reaction also worked well on a 10 mmol scale and was used for the direct synthesis of the agrochemicals iprobenfos and inezin, proving its practical utility.
The team carried out experiments to investigate the reaction mechanism. Without irradiation, the yield of the reaction was much lower. The presence of a base was also essential. After addition of the free radical (2,2,6,6-tetramethylpiperidin-1-yl)oxyl (TEMPO), only traces of the target product and the TEMPO adduct of the disulfide component were detected. These experiments indicate that the reaction proceeds via a light- and base-mediated radical pathway.
- Blue LED-Promoted Syntheses of Phosphorothioates and Phosphorodithioates,
Bo-Ru Shen, Pratheepkumar Annamalai, Shih-Fang Wang, Rekha Bai, Chin-Fa Lee,
J. Org. Chem. 2022.
https://doi.org/10.1021/acs.joc.2c00323