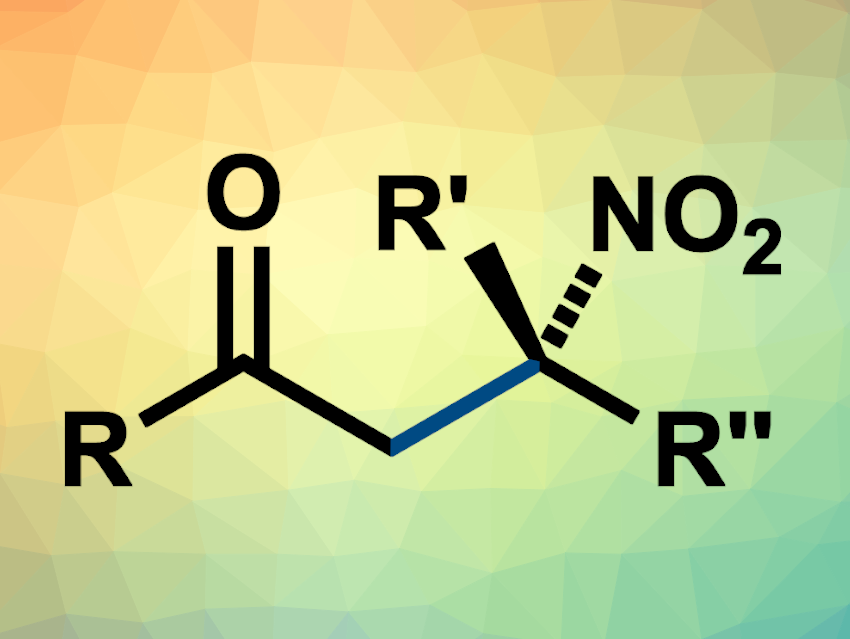
α-Tertiary amines are often found in pharmaceutically active compounds or natural products. They can be easily obtained from the corresponding tertiary nitroalkanes via a reduction reaction. Thus, approaches to the stereoselective preparation of tertiary nitroalkanes are useful. One possibility is the C-alkylation of secondary nitronates using alkyl halides. However, this reaction is challenging to achieve in a chemo- and stereoselective manner.
Todd K. Hyster, Cornell University, Ithaca, NY, USA, and colleagues have developed a chemo- and stereoselective C-alkylation of nitroalkanes with alkyl halides that is catalyzed by an engineered enzyme under cyan-colored LED irradiation. The team started from a flavin-dependent “ene”-reductase, i.e., old yellow enzyme from Geobacillus kaustophilus (GkOYE), and used directed evolution to obtain an enzyme that is well-suited to the desired reaction.
The researchers found a triple mutant called GkOYE-G7 that delivered the desired product (general structure pictured) with high yields and enantioselectivities. In addition, the enzyme retained its natural reductive reactivity. This allowed the team to create an enzymatic cascade reaction in which nitroalkenes are both reduced and alkylated to give chiral tertiary nitroalkanes. The resulting tertiary nitroalkanes could be reduced to the corresponding α-tertiary amines without a loss of stereoselectivity.
- Asymmetric C-Alkylation of Nitroalkanes via Enzymatic Photoredox Catalysis,
Haigen Fu, Tianzhang Qiao, Jose M. Carceller, Samantha N. MacMillan, Todd K. Hyster,
J. Am. Chem. Soc. 2023.
https://doi.org/10.1021/jacs.2c12197