Healthcare-associated infections are a common problem in wound care, as is the rise in multi-drug resistant bacteria. In order to effectively and selectively combat bacterial infections, Subinoy Rana, Mrinmoy De, Indian Institute of Science, Bengaluru, India, and colleagues have developed a bactericidal nanomaterial equipped with a photochemical switch that can be directed either against Gram-positive or Gram-negative bacteria. The material showed efficacy against MRSA, and its activity could potentially also be extended to other bacterial strains.
Bactericidal Materials
Antibiotic-resistant infections have become an important public health concern, particularly in hospital settings. Many of the bacterial species in question are widespread in Nature, but can cause serious, sometimes untreatable, infections in immunocompromised patients.
Bactericidal materials offer a new approach to combating healthcare-associated infections that does not rely on antibiotics. The team developed a UV/visible-light-responsive nanomaterial that can be switched to target either Gram-positive or Gram-negative bacteria.
Gram-Positive and Gram-Negative Bacteria
Both bacteria types have very different outer membrane structures and compositions. Gram-positive bacteria, including methicillin-resistant Staphylococcus aureus (MRSA), have a bacterial membrane majorly composed of peptidoglycans.
In contrast, Gram-negative bacteria, including Pseudomonas aeruginosa, another healthcare-associated bacterium with problematic resistance to broadband antibiotics, has both inner and outer membranes mainly composed of phospholipids with a thin peptidoglycan layer. “It is important to achieve strain-selective bactericidal activity,” De says.
Functionalized Nanomaterial with Light-Controlled Selectivity
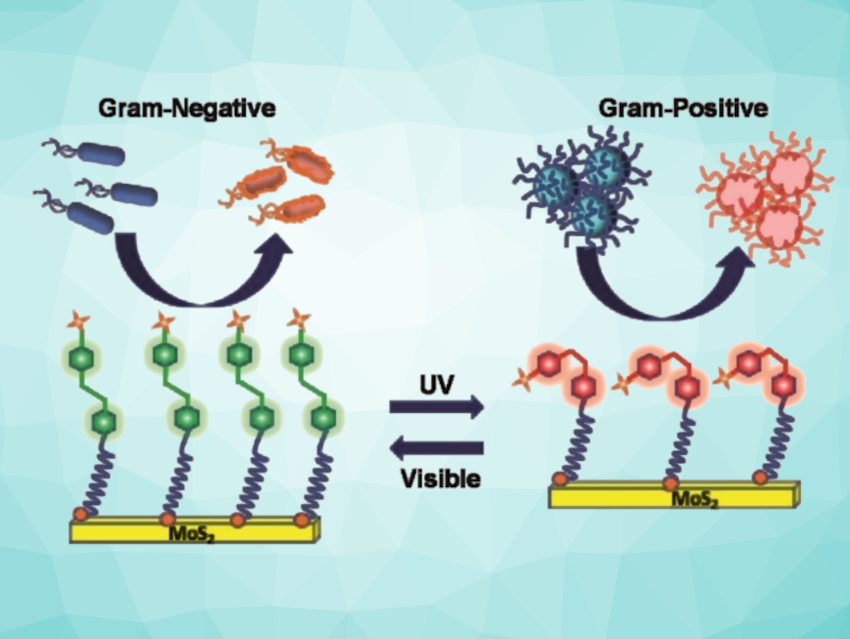
To achieve a bactericidal agent that could selectively interact with both chemical surfaces, the team designed a functionalized nanomaterial made of molybdenum disulfide sheets (2D-MoS2) with azobenzene moieties to which positively charged quaternary amino groups were attached.
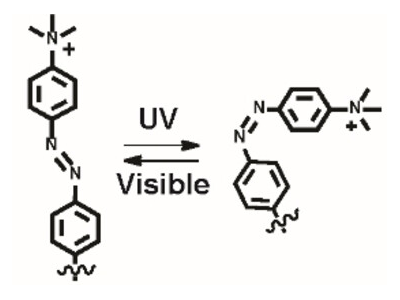
MoS2 is a bactericide and the quaternary amino groups allow membrane depolarization. The azobenzene moieties introduce a light-driven switch in the nanostructure from an elongated trans into a curved cis form (pictured below) to create selective surface interactions.
Antibacterial Activity
The team used chemical probes and optical measurements to study the effects of the nanomaterial and found that both the cis and trans forms killed bacteria, albeit in different ways. For the Gram-negative P. aeruginosa, the trans form depolarized the bacterial membrane and pierced it thoroughly. This allowed the MoS2 nanomaterial to generate intracellular reactive oxygen species and kill the bacteria.
Conversely, a Gram-positive MRSA strain responded to the cis form more effectively. In this case, the cell wall was damaged and ruptured by specific interactions. By simply “flipping” the photoswitch from the trans ground state to the cis state, the team was able to control selectivity for either bacterial type.
The researchers showed the efficacy of their nanomaterial by successfully healing MRSA-infected wounds in mice. Wounds treated with the cis reagent completely closed after ten days, which demonstrated faster wound healing compared with a conventional vancomycin treatment.
- Photo‐Controlled Gating of Selective Bacterial Membrane Interaction and Enhanced Antibacterial Activity for Wound Healing,
Jagabandhu Sahoo, Soumyashree Sahoo, Yogeswari Subramaniam, Preeti Bhatt, Subinoy Rana, Mrinmoy De,
Angew. Chem. Int. Ed. 2023.
https://doi.org/10.1002/anie.202314804