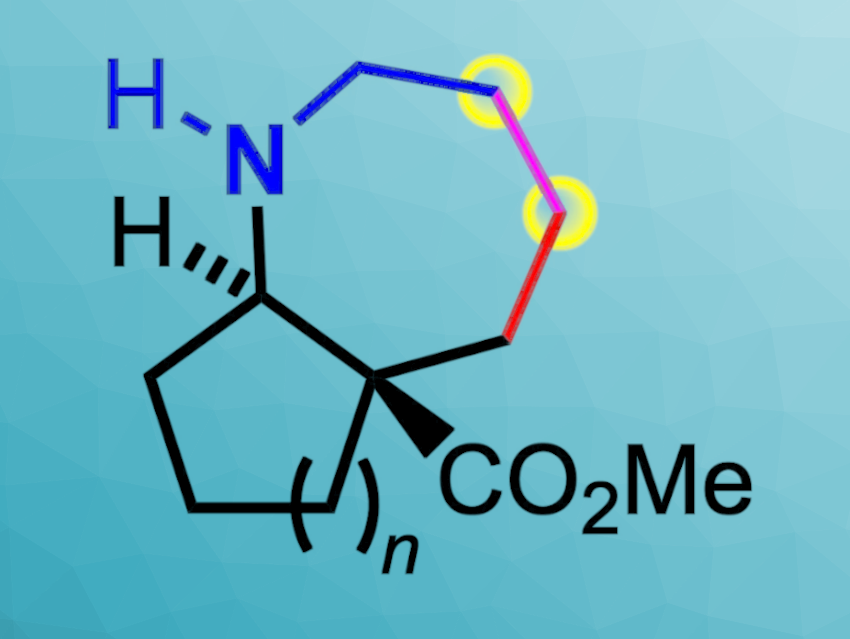
Annulated azepane rings (seven-membered rings with one nitrogen atom) are rare, but important structural motifs in natural products and active pharmaceutical ingredients. With at least two stereogenic centers, enantioselective access to such compounds could be useful for the development of new biologically active compounds.
Jens Christoffers, University of Oldenburg, Germany, and colleagues have developed a straightforward synthesis of optically active [b]-annulated azepane scaffolds via olefin cross-metathesis followed by exhaustive hydrogenation (pictured).
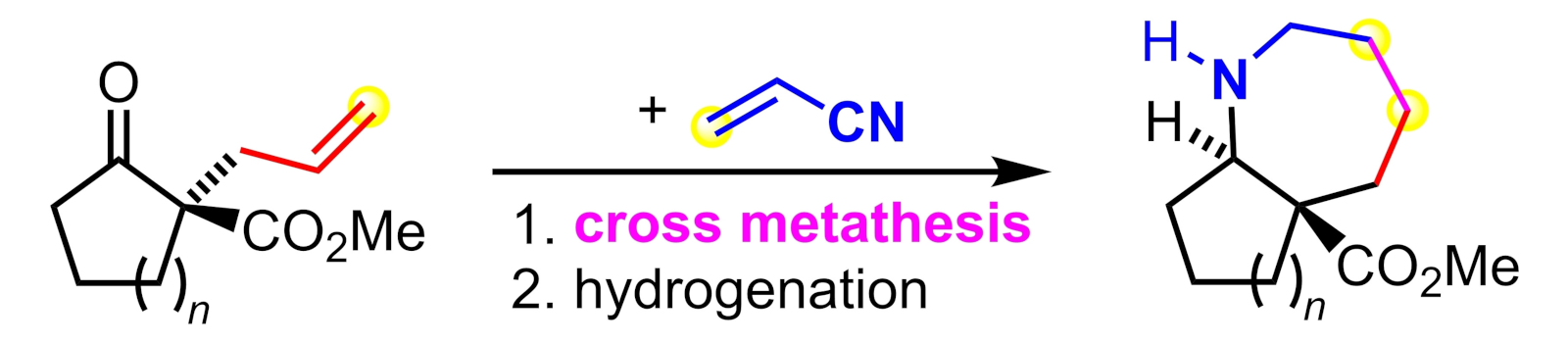
The team started with optically active, cyclic α-allyl-β-oxoesters, which were accessed by palladium-catalyzed asymmetric allylic alkylation. These compounds were submitted to ruthenium-catalyzed cross-metathesis with acrylonitrile (pictured in blue), which gives intermediate products with an ω-cyanoallyl side chain. Then a heterogeneous palladium-catalyzed hydrogenation of the C–C double and C–N triple bonds was performed, followed by reductive amination via an iminium ion formed in situ from the primary amino function and the endocyclic carbonyl group. This key step gave the desired annulated azepanes with relative trans-configuration in a stereoselective manner.
The amino function and the ester group of the synthesized cyclopenta-, benzo-, and cyclohepta[b]-annulated azepanes offer two possible points for further diversification. The team explored both by the preparation of several derivatives.
- Enantioselective Synthesis of [b]‐Annulated Azepane Scaffolds ,
Enno Aeissen, Aaron R. von Seggern, Marc Schmidtmann, Jens Christoffers,
Eur. J. Org. Chem. 2023.
https://doi.org/10.1002/ejoc.202300180