Promethium (Pm), discovered in 1945, is the only element in the lanthanide series without stable isotopes. Nowadays, the synthetic radioisotope 147Pm with a half-life of τ1/2 = 2.62 years can be produced in small quantities by nuclear fission and extensive purification processes. The rare earth element is used, for example, in long-life nuclear batteries for spacecraft and in radiation therapy.
To date, only a handful of promethium compounds have been prepared, all of which are simple solids such as an oxide. A complex that shows how promethium could bind to ligands in solution, for example, to separate it from other rare earth elements, has not yet been found.
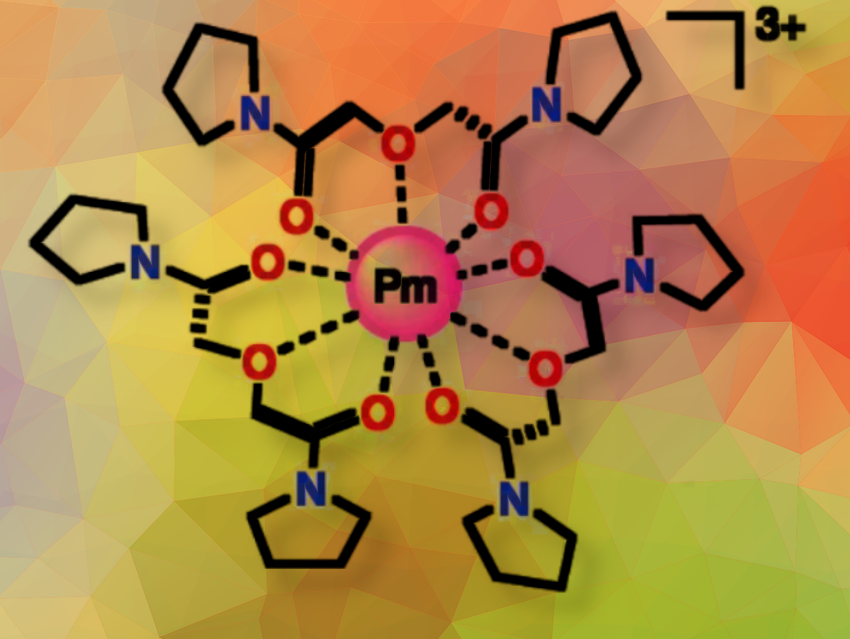
Ilja Popovs, Alexander S. Ivanov, and colleagues, Oak Ridge National Laboratory, TN, USA, have studied how the Pm3+ ion interacts with a multidentate ligand in water. They used improved isotope separation techniques to produce enough of the synthetic radioisotope 147Pm for these studies. Then they synthesized a new water-soluble complexing agent, bispyrrolidine diglycolamide (PyDGA), and used it for Pm complexation.
Using X-ray absorption spectroscopy (XAS) at the National Synchrotron Light Source II (NSLS-II) and theoretical simulations, the researchers measured the average bond length of the homoleptic [Pm(PyDGA)3]3+ complex (pictured) and found that oxygen atoms form bonds with Pm by donating pairs of electrons that occupy the empty orbitals around the Pm ion, thereby stabilizing the complex.
The researchers then synthesized comparable complexes of all lanthanides using the same ligand. Thereby they found that the lanthanide–oxygen bond lengths decrease from left to right across the lanthanide series due to the lanthanide contraction effect. As one moves from lanthanum to lutetium in the lanthanide series, the increasing nuclear charge due to added protons and electrons, particularly filling the diffuse 4f orbitals that provide poor shielding, causes a stronger nuclear pull on the electrons, leading to the phenomenon known as lanthanide contraction.
The researchers also observed that the decrease in bond length in the earlier part of the series, from lanthanum to promethium, was sharper than in the later part of the series.
- Observation of a promethium complex in solution,
Darren M. Driscoll, Frankie D. White, Subhamay Pramanik, Jeffrey D. Einkauf, Bruce Ravel, Dmytro Bykov, Santanu Roy, Richard T. Mayes, Lætitia H. Delmau, Samantha K. Cary, Thomas Dyke, April Miller, Matt Silveira, Shelley M. VanCleve, Sandra M. Davern, Santa Jansone-Popova, Ilja Popovs, Alexander S. Ivanov,
Nature 2024, 629, 819–823.
https://doi.org/10.1038/s41586-024-07267-6