Phosphines are useful, e.g., in catalysis, materials science, chemical synthesis, and medicinal chemistry. However, many phosphines are sensitive to oxidation and unstable in air. To safeguard against this, researchers have developed two proven yet tedious strategies: working under an oxygen-free inert atmosphere or attaching bulky substituents.
Filip Horký, Rudolf Pietschnig, University of Kassel, Germany, and colleagues have developed a series of primary phosphines displaying remarkable stability in air. The phosphines of the type Fc(CH2)nPH2 (n = 0,1,2,3; Fc = ferrocenyl) were synthesized according to known procedures and then oxidized using air or H2O2 to obtain the corresponding isolable primary phosphine oxides. The team found that the compounds were surprisingly air-stable.
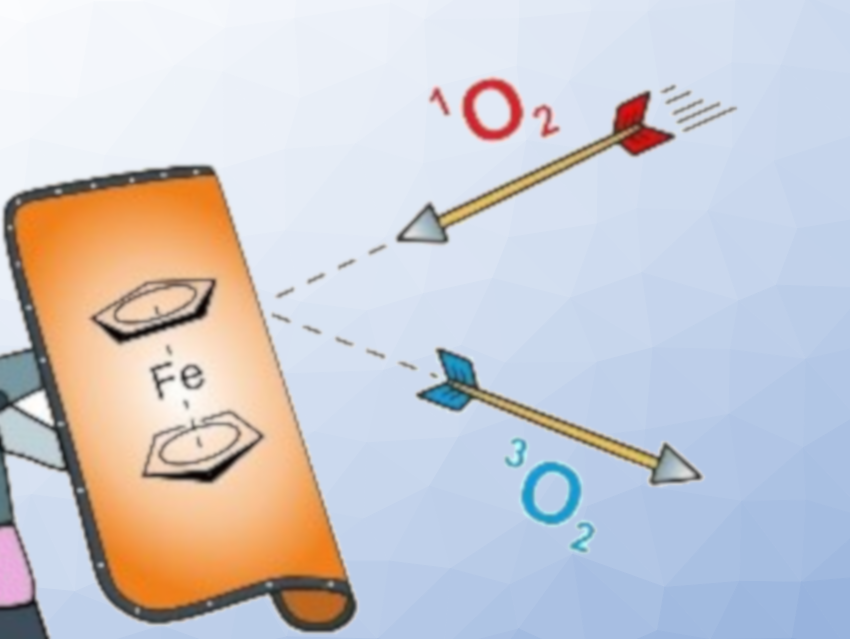
The team found that the ferrocene unit exerts an antioxidant effect on the primary phosphine group, quenching singlet oxygen (pictured). In addition, the presence of ferrocene in solution also inhibits the oxidation of other secondary and tertiary phosphines in air, i.e., ferrocene can provide intermolecular stabilization. The quenching of singlet oxygen as the reason for the antioxidant effect which was confirmed using other known singlet-oxygen quenchers. This result opens a new strategy for enhancing the air stability of phosphines. The strategy works even with as little as 1 % of a quencher, which is not consumed, unlike classical antioxidants.
- A General Strategy for Increasing the Air‐stability of Phosphines Including Primary Phosphines,
Filip Horký, Roman Franz, Clemens Bruhn, Rudolf Pietschnig,
Chem. Eur. J. 2023.
https://doi.org/10.1002/chem.202302518