Chemistry is increasingly making use of the “trick” plants use for photosynthesis: driving chemical reactions that run poorly or do not occur spontaneously at all with light energy. This requires suitable photocatalysts that can capture light energy and make it available for the reaction. Kaifeng Wu, Dalian Institute of Chemical Physics, Chinese Academy of Sciences, and University of the Chinese Academy of Sciences, Beijing, and colleagues have introduced layered core/shell quantum dots that efficiently drive challenging organic transformations. Their low toxicity is a particular advantage.
ZnSe/ZnS Quantum Dots
Quantum dots are finely dispersed nanoscopic crystals of inorganic semiconductors. They absorb strongly in an adjustable range of the spectrum and are easy to recycle. Until now, photocatalytic quantum dots have been based almost exclusively on the highly toxic elements cadmium and lead. This fact and their limited efficiency have been the main barriers to their broader use.
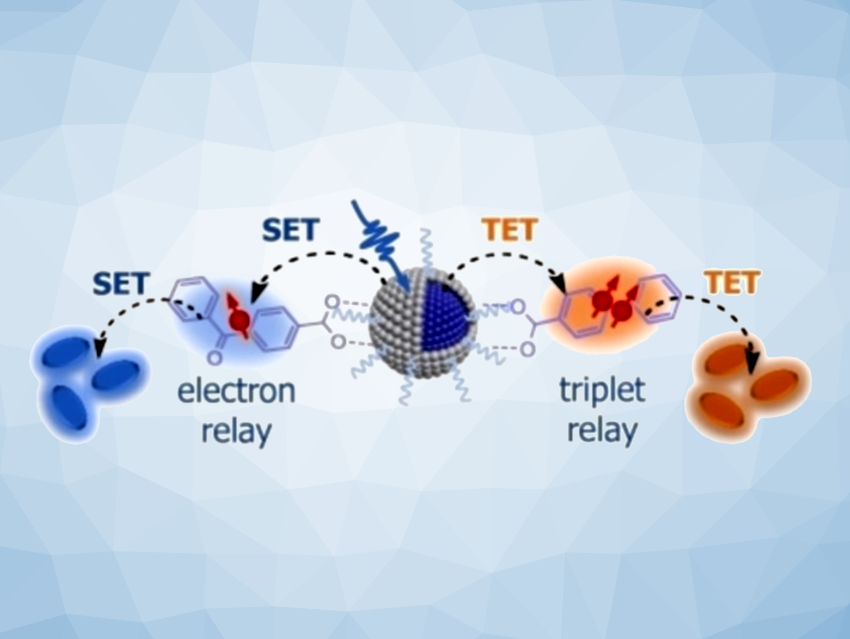
The team has introduced novel quantum dots with low toxicity and high performance. They are activated by commercially available blue LEDs—the UV light that is usually required is not needed. The secret to their success lies in their core/shell structure and the variable coatings that can be used to “store” the light energy.
The quantum dots are only a few nanometers wide. Their core consists of zinc selenide (ZnSe) and is surrounded by a thin shell made of zinc sulfide (ZnS). Blue light raises the zinc selenide to an excited state in which it can easily give up electrons. The shell prevents the electrons from immediately being captured by so-called defects.
Useful Tools for Organic Transformations
The team equipped the surface of the shell with special benzophenone ligands that “suck up” the electrons from the quantum dots, store them, and make them available for organic reactions. For example, the researchers were able to carry out reductive dehalogenations of aryl chlorides and additive-free polymerizations of acrylates—important reactions that run poorly or not at all when using conventional photocatalysts.
A second version was made by coating the surface with biphenyl ligands that can directly absorb energy from excited quantum dots. This brings them into a long-lived, highly energetic triplet state. The triplet energy “stored” in this way can be transferred to specific organic molecules, which then also enter a triplet state. In this state, they can undergo chemical reactions that are not possible in their ground state. As a demonstration, the team carried out [2+2] homo-cycloadditions of styrene and cycloadditions of carbonyls with alkenes. These produce four-membered rings (cyclobutanes or oxetanes, respectively), which are substances that are important starting materials in areas such as pharmaceutical development.
- Low‐Toxicity ZnSe/ZnS Quantum Dots as Potent Photoreductants and Triplet Sensitizers for Organic Transformations,
Chengming Nie, Xuyang Lin, Guohui Zhao, Kaifeng Wu,
Angew. Chem. Int. Ed. 2022.
https://doi.org/10.1002/anie.202213065