Carboranes are clusters composed of boron, carbon, and hydrogen atoms. They have applications, e.g., in coordination chemistry, catalysis, optoelectronics, and supramolecular chemistry. Methods for the synthesis of functionalized carboranes are, thus, useful. However, the selective functionalization of o-carboranes—for example, the introduction of nitrogen-containing functional groups at boron—can be challenging.
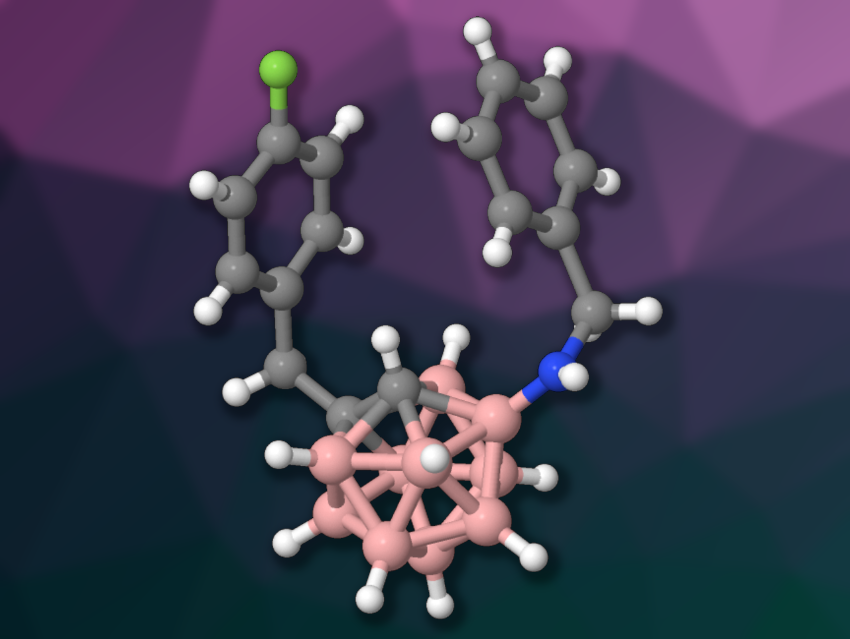
Phil Ho Lee, Kangwon National University, Chuncheon, Republic of Korea, and colleagues have developed an Ir(III)-catalyzed, decarboxylative, regioselective B(4)–H amination reaction of o-carborane acids (example product pictured). The team used sulfilimines as aminating reagents. They reacted different C(2)-alkyl-substituted o-carborane acids with N-arylsulfonyl sulfilimines or N-methanesulfonyl sulfilimine, using [Cp*IrCl2]2 as the catalyst together with AgSbF6 and KOAc in 1,2-dichloroethane (DCE) as the solvent. For N-alkyl sulfilimines, the team used modified reaction conditions with [Cp*IrCl2]2, AgNO3, and NEt3 in hexafluoroisopropanol (HFIP). The reactions were performed at 60 °C under air.
The desired B(4)-aminated o-carboranes were obtained in mostly good to excellent yields. The team proposes a reaction mechanism that involves the formation of a catalytically active cationic Ir(III) species, which reacts with the substrate to give an iridacycle. This intermediate reacts with the sulfilimine, forming an Ir(V) nitrene complex. This is followed by insertion of the nitrene into the B–Ir bond, protonation, and decarboxylation to give the desired product. Overall, the work provides access to a broad range of B(4)–H aminated o-carboranes.
- Iridium(III)-Catalyzed Regioselective B(4)–H Amination of o-Carboranes with Sufilimines,
Kyeongna Park, Gi Uk Han, Sugyeong Yoon, Eunseo Lee, Hee Chan Noh, Kyungsup Lee, Chanyoung Maeng, Dongwook Kim, Phil Ho Lee,
Org. Lett. 2023.
https://doi.org/10.1021/acs.orglett.3c02114