α-Aminonitriles are useful intermediates in organic synthesis. They can be synthesized, e.g., via an α-C–H functionalization of amines. Usually, this approach is used with protected or tertiary amines as substrates. The α-cyanation of unprotected secondary amines is less well studied.
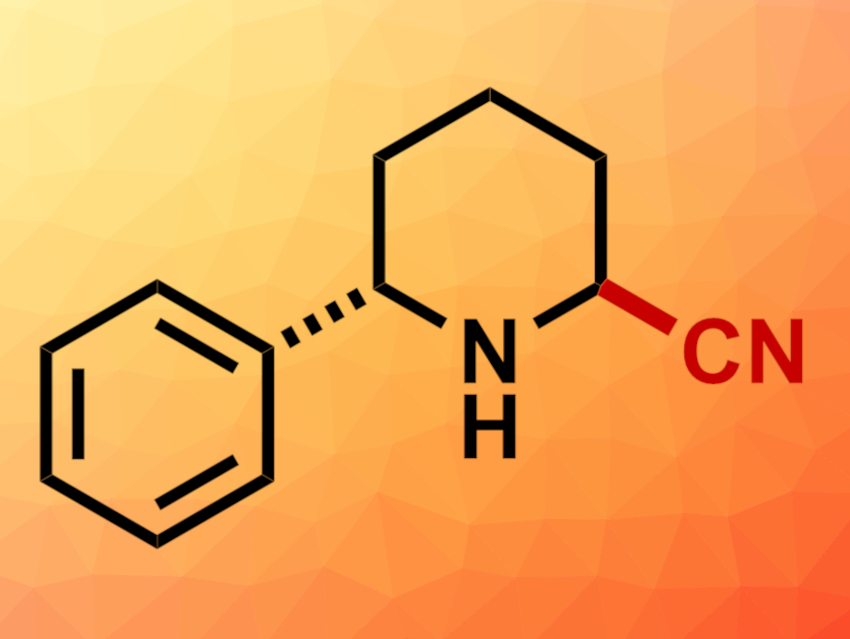
Daniel Seidel, University of Florida, Gainesville, USA, and colleagues have developed a method for the regioselective α’-cyanation of unprotected cyclic amines that have a pre-existing substituent in the other α position (example product pictured). The C–H bonds at the unsubstituted α’ site in these amines are usually less reactive than the C–H bond at the substituted α site, which makes this selectivity challenging to achieve.
The team first converted the amine substrates to lithium amides using n-BuLi. The resulting intermediate was oxidized using trifluoroacetophenone as a ketone oxidant, giving an imine. Finally, the addition of trimethylsilyl cyanide (TMSCN) to the imine gave the desired α-aminonitriles.
The products were obtained in moderate to good yields. The reaction showed a broad scope and proved suitable for the α-cyanation of simple amines such as pyrrolidine or piperidine and for the α’-cyanation of a range of amines with an existing α-substituent. The obtained α-aminonitriles can be further converted, e.g., into the corresponding α-amino acids.
- Regioselective α-Cyanation of Unprotected Alicyclic Amines,
Fuchao Yu, Daniel A. Valles, Weijie Chen, Scott D. Daniel, Ion Ghiviriga, Daniel Seidel,
Org. Lett. 2022.
https://doi.org/10.1021/acs.orglett.2c02148


![A Path to Substituted Bicyclo[2.1.1]hexanones](https://www.chemistryviews.org/wp-content/uploads/2024/10/1substitutedbicyclo211hexan2ones_2024-125x94.png)
