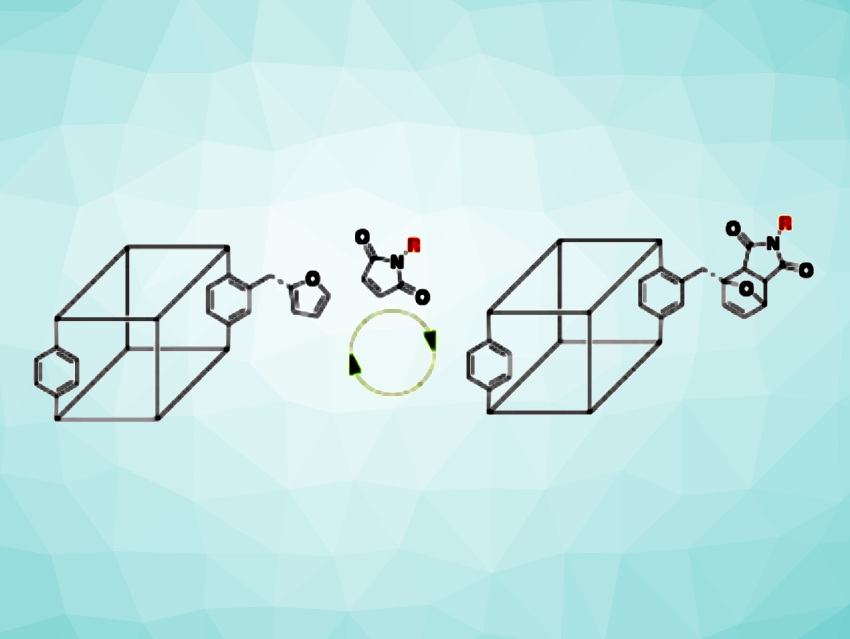
Metal–organic frameworks (MOFs) are crystalline, porous materials composed of metal centers and organic linkers. They have applications, e.g., in catalysis or gas separation. Postsynthetic modification can be used to incorporate different functional groups in MOFs to tailor their properties. Typically, covalent postsynthetic modification installs functional groups in a non-reversible manner.
Seth M. Cohen, University of California, San Diego, La Jolla, USA, and colleagues have developed a method for the reversible postsynthetic modification of MOFs using a furan/maleimide Diels-Alder reaction, maintaining the integrity of the MOF. The team prepared a Zn(II)-based framework that is functionalized with azidomethyl groups (called IRMOF-(CH2N3)20) using a mixture of the organic linkers 2-azidomethylterephthalic acid (H2bdcCH2N3, bdc = 1,4-benzenedicarboxylate) and terephthalic acid (H2bdc). The azido groups were then used to functionalize the MOF with furan units via a copper-catalyzed click reaction with 2-((prop-2-yn-1-yloxy)methyl)furan.
The resulting furan-functionalized MOF was reacted with maleimides such as N-(2-hydroxyethyl)maleimide at 60 °C. The team found that the maleimide reacted with the furan groups in a Diels–Alder reaction with an estimated conversion of 85 %. Heating the resulting MOF to 100 °C in dimethylformamide (DMF) for 24 h allowed the researchers to reverse this reaction, restoring the original furan-containing framework. The porosity and crystallinity of the MOF was retained in this process, and the team showed that the same MOF sample can be used in multiple rounds of Diels–Alder-based functionalization/defunctionalization. The team’s approach could be useful, e.g., for the development of stimuli-responsive MOFs.
- Reversible Postsynthetic Modification in a Metal‐Organic Framework,
Prantik Mondal, Zachary Neuschuler, Dipendu Mandal, Ritchie E Hernandez, Seth Mason Cohen,
Angew. Chem. Int. Ed. 2023.
https://doi.org/10.1002/anie.202317062