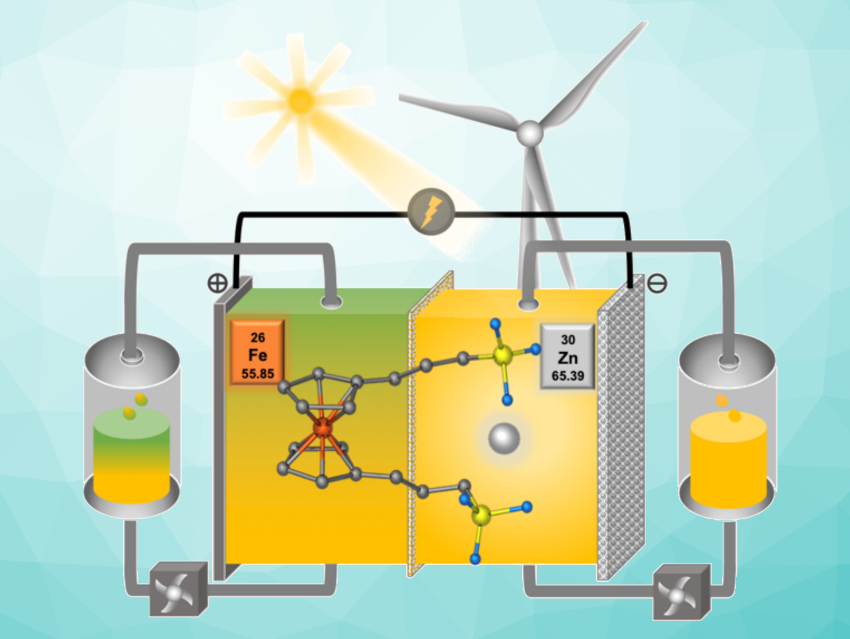
Redox flow batteries (RFBs) could be a promising option for scalable renewable energy storage. Zinc is an attractive anode material for aqueous redox flow batteries (ARFBs) due to its low cost and high capacity. However, applications of traditional inorganic Zn halide flow batteries are hampered by the properties of halide cathode electrolytes, e.g., corrosion and toxicity. These issues could be addressed by using sustainable, redox-active organic molecules as cathode electrolytes.
Tianbiao Leo Liu and colleagues, Utah State University, Logan, USA, have developed a bipolar redox-active zinc 1,1′-bis(3-sulfonatopropyl)ferrocene (Zn[Fc(SPr)2]) electrolyte for ARFBs (pictured above). The electrolyte was synthesized from ferrocene via a three-step reaction, and its physicochemical properties and battery performance were investigated by NMR and IR spectroscopy as well as electrochemical studies.
A Zn-Fc ARFB with 20.2 Wh/L energy density displayed nearly 100 % capacity retention for 2,000 cycles over ca. 53 days. The bipolar design avoids the undesired crossover of redox-active species typically caused by imperfect ion-selective membranes, which has a negative impact on the battery cycling stability. It also reduces energy-storage costs by enabling compatibility with a low-cost porous membrane. The researchers believe that the bipolar electrolyte design could be extended to other high-performance redox materials for flow batteries and electrochemical devices.
- An Energy Dense, Powerful, Robust Bipolar Zinc‐Ferrocene Redox Flow Battery,
Jian Luo, Bo Hu, Maowei Hu, Wenda Wu, Tianbiao Leo Liu,
Angew. Chem. Int. Ed. 2022.
https://doi.org/10.1002/anie.202204030